当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Steps control the dissociation of CO2 on Cu(100)
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-18 , DOI: 10.1021/jacs.8b07906 Benjamin Hagman 1 , Alvaro Posada-Borbón , Andreas Schaefer , Mikhail Shipilin 2 , Chu Zhang 1 , Lindsay R. Merte 3 , Anders Hellman , Edvin Lundgren 1 , Henrik Grönbeck , Johan Gustafson 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-18 , DOI: 10.1021/jacs.8b07906 Benjamin Hagman 1 , Alvaro Posada-Borbón , Andreas Schaefer , Mikhail Shipilin 2 , Chu Zhang 1 , Lindsay R. Merte 3 , Anders Hellman , Edvin Lundgren 1 , Henrik Grönbeck , Johan Gustafson 1
Affiliation
|
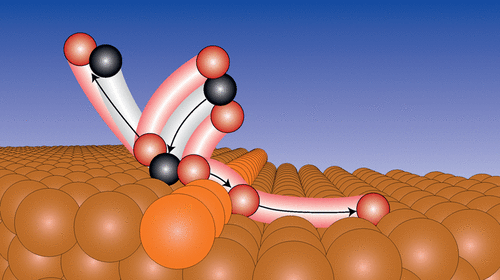
CO2 reduction reactions, which provide one route to limit the emission of this greenhouse gas, are commonly performed over Cu-based catalysts. Here, we use ambient pressure X-ray photoelectron spectroscopy together with density functional theory to obtain an atomistic understanding of the dissociative adsorption of CO2 on Cu(100). We find that the process is dominated by the presence of steps, which promote both a lowering of the dissociation barrier and an efficient separation between adsorbed O and CO, reducing the probability for recombination. The identification of steps as sites for efficient CO2 dissociation provides an understanding that can be used in the design of future CO2 reduction catalysts.
中文翻译:

步骤控制 CO2 在 Cu(100) 上的离解
CO2 还原反应提供了一种限制这种温室气体排放的途径,通常在铜基催化剂上进行。在这里,我们使用环境压力 X 射线光电子能谱和密度泛函理论来获得对 CO2 在 Cu(100) 上的解离吸附的原子理解。我们发现该过程由步骤的存在决定,这促进了解离势垒的降低和吸附的 O 和 CO 之间的有效分离,从而降低了重组的可能性。确定步骤作为有效 CO2 离解位点提供了一种理解,可用于设计未来的 CO2 还原催化剂。
更新日期:2018-09-18
中文翻译:

步骤控制 CO2 在 Cu(100) 上的离解
CO2 还原反应提供了一种限制这种温室气体排放的途径,通常在铜基催化剂上进行。在这里,我们使用环境压力 X 射线光电子能谱和密度泛函理论来获得对 CO2 在 Cu(100) 上的解离吸附的原子理解。我们发现该过程由步骤的存在决定,这促进了解离势垒的降低和吸附的 O 和 CO 之间的有效分离,从而降低了重组的可能性。确定步骤作为有效 CO2 离解位点提供了一种理解,可用于设计未来的 CO2 还原催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号