Science of the Total Environment ( IF 8.2 ) Pub Date : 2018-09-17 , DOI: 10.1016/j.scitotenv.2018.09.200 Jiani Xu , Yawen Liu , Fei Tao , Yifei Sun
|
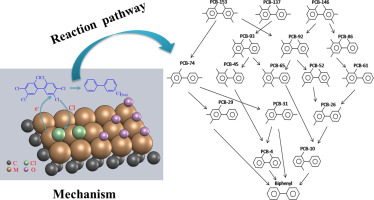
Bimetallic catalysts supported on activated carbon (AC) with high metal loadings were prepared by an ion-exchange method. AC-supported Ni-Cu, Ni-Zn and Ni-Pd bimetallic catalysts were used to decompose Aroclor 1254, which is one of the most commonly used commercial mix of polychlorinated biphenyls. Characterization by scanning electron microscopy and energy-dispersive X-ray analysis showed that the metals were uniformly distributed on the surfaces and inside the catalysts. The efficiencies of Aroclor 1254 decomposition were measured at different reaction temperatures and times. With increasing temperature, the catalytic activities increased and the activation energies of the reactions decreased, resulting in higher decomposition efficiencies. At 300 °C in a nitrogen atmosphere, Aroclor 1254 decomposition efficiencies of 99.3%, 99.4% and 99.5% were achieved for reactions with Ni-Cu/C, Ni-Zn/C and Ni-Pd/C, respectively. The kinetics and pathway of the decomposition reaction were discussed, and we concluded that the reactivity of the chlorine atoms located on the benzene rings followed the order para-position > meta-position > ortho-position. The PCBs were dechlorinated stepwise to form the final biphenyl product. The design concept and synthetic strategy developed in this study are of great significance in the disposal of chlorinated organic compounds, for use with the existing adsorption technology of AC.
中文翻译:

活性炭负载新型双金属催化剂去除Aroclor 1254的动力学和反应途径
采用离子交换法制备了载有高金属负载量的活性炭(AC)上的双金属催化剂。AC负载的Ni-Cu,Ni-Zn和Ni-Pd双金属催化剂被用来分解Aroclor 1254,它是最常用的多氯联苯工业混合物之一。通过扫描电子显微镜和能量色散X射线分析表征表明,金属均匀地分布在催化剂的表面和内部。在不同的反应温度和时间下测量了Aroclor 1254的分解效率。随着温度的升高,催化活性增加,反应的活化能降低,导致更高的分解效率。在氮气氛下,在300°C下,与Ni-Cu / C,Ni-Zn / C和Ni-Pd / C的反应分别达到99.3%,99.4%和99.5%的Aroclor 1254分解效率。讨论了分解反应的动力学和途径,得出结论,位于苯环上的氯原子的反应性依次为对位>间位>邻位。的多氯联苯进行脱氯逐步以形成最终的联苯产物。本研究中开发的设计概念和合成策略对于处理含氯有机化合物以及与现有的AC吸附技术一起使用具有重要意义。

































 京公网安备 11010802027423号
京公网安备 11010802027423号