Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigating Subcellular Compartment Targeting Effect of Porous Coordination Cages for Enhancing Cancer Nanotherapy
Small ( IF 13.0 ) Pub Date : 2018-09-17 , DOI: 10.1002/smll.201802709 Yu Fang 1 , Xizhen Lian 1 , Yanyan Huang 2 , Guo Fu 3 , Zhifeng Xiao 1 , Qi Wang 1 , Beiyan Nan 3 , Jean-Philippe Pellois 4 , Hong-Cai Zhou 1
Small ( IF 13.0 ) Pub Date : 2018-09-17 , DOI: 10.1002/smll.201802709 Yu Fang 1 , Xizhen Lian 1 , Yanyan Huang 2 , Guo Fu 3 , Zhifeng Xiao 1 , Qi Wang 1 , Beiyan Nan 3 , Jean-Philippe Pellois 4 , Hong-Cai Zhou 1
Affiliation
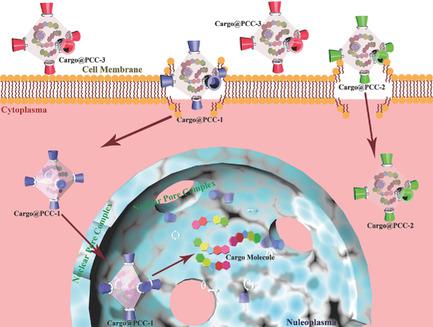
|
Understanding the key factors for successful subcellular compartment targeting for cargo delivery systems is of great interest in a variety of fields such as bionanotechnology, cell biology, and nanotherapies. However, the fundamental basis for intracellular transportation with these systems has thus far rarely been discussed. As a cargo vector, porous coordination cages (PCCs) have great potential for use in cancer nanotherapy and to elucidate fundamental insight regarding subcellular compartment targeting. Herein, it is shown that the transportation of PCC cargo vectors though various subcellular barriers of the mammalian cell can be manipulated by tuning the vector's electronic property and surface affinity. It is found that the PCCs become selectively aggregated at the cell membrane, the cytoplasm, or the nucleus, respectively. When a DNA topoisomerase inhibitor is delivered into the nucleus by a neutral and lipophilic PCC, the anticancer efficacy is dramatically improved. The findings shed light to tune the interactions at the “bio‐nano” interface. This study provides a key strategy for future work in targeting specific cell organelles for cell imaging, cargo delivery, and therapy. This research also offers key insight into the engineering of nanoscopic materials for furnishing cell organelle‐specificity.
中文翻译:

研究多孔配位笼的亚细胞区室靶向效应以增强癌症纳米治疗
了解货物输送系统成功实现亚细胞区室靶向的关键因素在生物纳米技术、细胞生物学和纳米疗法等多个领域引起了极大的兴趣。然而,迄今为止,很少讨论这些系统的细胞内运输的基本基础。作为一种货物载体,多孔配位笼(PCC)在癌症纳米疗法中具有巨大的应用潜力,并可阐明有关亚细胞区室靶向的基本见解。本文表明,PCC 货物载体通过哺乳动物细胞的各种亚细胞屏障的运输可以通过调整载体的电子特性和表面亲和力来操纵。研究发现PCCs分别选择性地聚集在细胞膜、细胞质或细胞核处。当DNA拓扑异构酶抑制剂通过中性亲脂性PCC递送到细胞核中时,抗癌功效显着提高。这些发现为调整“生物纳米”界面的相互作用提供了线索。这项研究为未来针对特定细胞器进行细胞成像、货物递送和治疗的工作提供了关键策略。这项研究还为提供细胞器特异性的纳米材料工程提供了重要见解。
更新日期:2018-09-17
中文翻译:

研究多孔配位笼的亚细胞区室靶向效应以增强癌症纳米治疗
了解货物输送系统成功实现亚细胞区室靶向的关键因素在生物纳米技术、细胞生物学和纳米疗法等多个领域引起了极大的兴趣。然而,迄今为止,很少讨论这些系统的细胞内运输的基本基础。作为一种货物载体,多孔配位笼(PCC)在癌症纳米疗法中具有巨大的应用潜力,并可阐明有关亚细胞区室靶向的基本见解。本文表明,PCC 货物载体通过哺乳动物细胞的各种亚细胞屏障的运输可以通过调整载体的电子特性和表面亲和力来操纵。研究发现PCCs分别选择性地聚集在细胞膜、细胞质或细胞核处。当DNA拓扑异构酶抑制剂通过中性亲脂性PCC递送到细胞核中时,抗癌功效显着提高。这些发现为调整“生物纳米”界面的相互作用提供了线索。这项研究为未来针对特定细胞器进行细胞成像、货物递送和治疗的工作提供了关键策略。这项研究还为提供细胞器特异性的纳米材料工程提供了重要见解。















































 京公网安备 11010802027423号
京公网安备 11010802027423号