Tetrahedron ( IF 2.1 ) Pub Date : 2018-09-15 , DOI: 10.1016/j.tet.2018.08.025 Ganesh Pandey , Jagadish Khamrai , Akash Mishra , Pulak Maity , Prasanna Kumara Chikkade
|
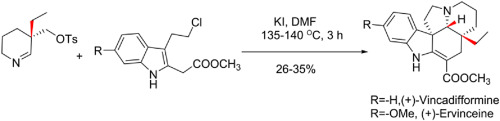
Asymmetric total synthesis of (+)-vincadifformine and (+)-ervinceine is reported by utilizing an iminium ion triggered cascade reaction using chiral 3,3-disubstituted piperidine imine and 2,3-disubstituted indole derivatives coupling partner. In the first generation total synthesis, the pivotal imine is prepared in excellent stereoselectivity but in moderate yield involving a Birch reduction-alkylation strategy. Furthermore, to improve upon the synthesis of the decisive imine, the more practical second generation route is devised through a Johnson-Claisen rearrangement to access chiral 3,3-disubstituted piperidinone in excellent yield and stereoselectivity. Apart from synthesizing (+)-vincadifformine, this strategy is also exploited for the first asymmetric total synthesis of (+)-ervinceine employing an iminium ion mediated cascade reaction. This distinctive strategy simultaneously sets up two new rings, two new stereogenic centers, and three new sigma bonds in a single operation.
中文翻译:

亚胺离子-烯胺级联反应可实现曲霉精中生物碱长春花吗啡和ervinceine的不对称全合成
据报道,利用手性3,3-二取代的哌啶亚胺和2,3-二取代的吲哚衍生物偶合伙伴,通过亚胺离子触发的级联反应,报道了(+)-长春花碱和(+)-香豆素的不对称全合成。在第一代全合成中,关键的亚胺以优异的立体选择性但以适中的产率制备,涉及桦木还原烷基化策略。此外,为了改善决定性亚胺的合成,通过约翰逊-克莱森重排设计了更实用的第二代路线,以优异的产率和立体选择性接近手性3,3-二取代的哌啶酮。除了合成(+)-长春新碱外,这种策略还被用于通过亚胺离子介导的级联反应进行的第一个不对称全合成(+)-长春新碱。

































 京公网安备 11010802027423号
京公网安备 11010802027423号