当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation, Alkylation, and Hydrolysis of Chiral Nonracemic N-Amino Cyclic Carbamate Hydrazones: An Approach to the Enantioselective α-Alkylation of Ketones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-09-10 00:00:00 , DOI: 10.1021/acs.joc.8b00655 Uyen Huynh 1 , Stacey L. McDonald 1 , Daniel Lim 1 , Md. Nasir Uddin 1 , Sarah E. Wengryniuk 1 , Sumit Dey 1 , Don M. Coltart 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-09-10 00:00:00 , DOI: 10.1021/acs.joc.8b00655 Uyen Huynh 1 , Stacey L. McDonald 1 , Daniel Lim 1 , Md. Nasir Uddin 1 , Sarah E. Wengryniuk 1 , Sumit Dey 1 , Don M. Coltart 1
Affiliation
|
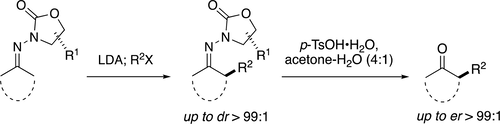
The α-alkylation of ketones is a fundamental synthetic transformation. The development of asymmetric variants of this reaction is important given that numerous natural products, drugs, and related compounds exist as α-functionalized ketones or derivatives thereof. We previously reported our preliminary studies on the development of a new enantioselective ketone α-alkylation procedure using N-amino cyclic carbamate (ACC) auxiliaries. In comparison to other auxiliary-based methods, ACC alkylation offers a number of advantages and is both highly enantioselective and high yielding. Herein, we provide a full account of our studies on the enantioselective ACC ketone α-alkylation method.
中文翻译:

手性非外消旋N-氨基环状氨基甲酸酯Hy的形成,烷基化和水解:酮的对映选择性α-烷基化方法
酮的α-烷基化是基本的合成转化。鉴于许多天然产物,药物和相关化合物以α-官能化的酮或其衍生物形式存在,因此开发该反应的不对称变体非常重要。我们以前曾报道过使用N-氨基环状氨基甲酸酯(ACC)助剂开发新的对映选择性酮α-烷基化程序的初步研究。与其他基于辅助方法的方法相比,ACC烷基化具有许多优势,并且具有高对映选择性和高收率。在此,我们全面介绍了对映选择性ACC酮α-烷基化方法的研究。
更新日期:2018-09-10
中文翻译:

手性非外消旋N-氨基环状氨基甲酸酯Hy的形成,烷基化和水解:酮的对映选择性α-烷基化方法
酮的α-烷基化是基本的合成转化。鉴于许多天然产物,药物和相关化合物以α-官能化的酮或其衍生物形式存在,因此开发该反应的不对称变体非常重要。我们以前曾报道过使用N-氨基环状氨基甲酸酯(ACC)助剂开发新的对映选择性酮α-烷基化程序的初步研究。与其他基于辅助方法的方法相比,ACC烷基化具有许多优势,并且具有高对映选择性和高收率。在此,我们全面介绍了对映选择性ACC酮α-烷基化方法的研究。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号