European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2018-09-07 , DOI: 10.1016/j.ejpb.2018.09.005 Isabell Speer , Denise Steiner , Yasmin Thabet , Jörg Breitkreutz , Arno Kwade
|
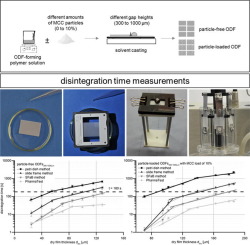
Orodispersible films (ODFs) provide high application comfort due to rapid disintegration in the oral cavity. They increasingly found the approval of pharmaceutical research and development and were added to the European Pharmacopeia in 2012. The European Pharmacopeia explicitly demands disintegration testing for ODFs, but does not refer to a suitable method. The aim of this study was to collect and evaluate existing disintegration methods regarding their suitability to investigate different ODF formulations. Therefore, 21 ODF formulations produced at different gap heights and/or different amounts of suspended microcrystalline cellulose (MCC) particles were manufactured by solvent casting technique, using hypromellose (HPMC) as film-forming polymer. Four disintegration methods described in literature were applied to characterize the disintegration behavior of these formulations. They were the petri dish, the slide frame, slide frame and ball (SFaB) method as well as the PharmaTest® disintegration tester equipped with a film sample holder. All methods show similar tendencies, at which the disintegration time proportionally increases with increasing dry film thicknesses. Reduced disintegration times were observed for ODFs containing insoluble MCC particles compared to their corresponding particle-free formulations. However, the suitability to investigate varying types of ODFs applying the four different test methods highly depends on the intended purpose. Therefore, the slide frame and SFaB method seems to be particularly applicable for research and development purposes, whereas the PharmaTest® disintegration tester and the SFaB method fulfil the demands required for testing methods within the quality control.
中文翻译:

口腔膜制剂崩解方法的比较研究
由于口腔中的快速崩解,可口分散的薄膜(ODF)提供了很高的应用舒适性。他们越来越多地获得药物研发的认可,并于2012年加入《欧洲药典》。《欧洲药典》明确要求对ODF进行崩解测试,但未提及合适的方法。这项研究的目的是收集和评估现有的崩解方法是否适合研究不同的ODF配方。因此,使用羟丙甲纤维素(HPMC)作为成膜聚合物,通过溶剂流延技术制备了以不同的间隙高度和/或不同数量的悬浮微晶纤维素(MCC)颗粒生产的21种ODF制剂。应用文献中描述的四种崩解方法来表征这些制剂的崩解行为。它们是培养皿,滑动架,滑动架和球(SFaB)方法以及PharmaTest®崩解测试仪配有薄膜样品架。所有方法都显示出相似的趋势,在这种趋势下,崩解时间会随着干膜厚度的增加而成比例地增加。与含有相应的无颗粒制剂相比,含有不溶性MCC颗粒的ODF的崩解时间缩短了。但是,使用四种不同的测试方法来研究不同类型的ODF的适用性在很大程度上取决于预期的目的。因此,滑动框架和SFAB方法似乎是特别适用于研究和开发的目的,而PharmaTest ®崩解测试仪和SFAB方法满足对质量控制中测试方法所需的要求。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号