当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
FeCl3-promoted tandem 1,4-conjugate addition/6-endo-dig cyclization/oxidation of propargylamines and benzoylacetonitriles/malononitriles: direct access to functionalized 2-aryl-4H-chromenes
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-08 , DOI: 10.1039/c8ob01927d Xinwei He 1, 2, 3, 4, 5 , Hui Wang 1, 2, 3, 4, 5 , Xiaoting Cai 1, 2, 3, 4, 5 , Qianqian Li 1, 2, 3, 4, 5 , Jiajia Tao 1, 2, 3, 4, 5 , Yongjia Shang 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-08 , DOI: 10.1039/c8ob01927d Xinwei He 1, 2, 3, 4, 5 , Hui Wang 1, 2, 3, 4, 5 , Xiaoting Cai 1, 2, 3, 4, 5 , Qianqian Li 1, 2, 3, 4, 5 , Jiajia Tao 1, 2, 3, 4, 5 , Yongjia Shang 1, 2, 3, 4, 5
Affiliation
|
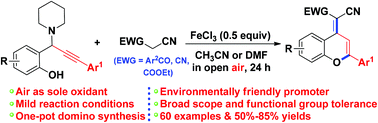
An efficient and concise procedure has been developed for the synthesis of functionalized 2-aryl-4H-chromenes based on a tandem reaction of propargylamines and benzoylacetonitriles/malononitriles in the presence of FeCl3 as an environmentally friendly promoter. This reaction involves a highly efficient tandem sequence consisting of 1,4-conjugate addition, 6-endo-dig cyclization, and oxidation. This protocol tolerates a variety of functional groups, thereby providing a practical and efficient method for the fabrication of 2-aryl-4H-chromene skeletons.
中文翻译:

FeCl 3促进的串联1,4-共轭加成/ 6-炔丙基胺和苯甲酰基乙腈/丙二腈的内挖环化/氧化:直接获得功能化的2-芳基-4 H-苯甲基
一种有效和简洁的方法已经为官能化的2-芳基4的合成已经开发ħ -chromenes基于炔丙胺和benzoylacetonitriles的串联反应/丙二腈在的FeCl存在3作为环保型启动子。该反应涉及由1,4-缀合物加成,6-内-挖-环化和氧化组成的高效串联序列。该方案可耐受多种官能团,从而为制造2-芳基-4 H-色烯骨架提供了一种实用而有效的方法。
更新日期:2018-10-11
中文翻译:

FeCl 3促进的串联1,4-共轭加成/ 6-炔丙基胺和苯甲酰基乙腈/丙二腈的内挖环化/氧化:直接获得功能化的2-芳基-4 H-苯甲基
一种有效和简洁的方法已经为官能化的2-芳基4的合成已经开发ħ -chromenes基于炔丙胺和benzoylacetonitriles的串联反应/丙二腈在的FeCl存在3作为环保型启动子。该反应涉及由1,4-缀合物加成,6-内-挖-环化和氧化组成的高效串联序列。该方案可耐受多种官能团,从而为制造2-芳基-4 H-色烯骨架提供了一种实用而有效的方法。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号