当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fe(II)-Catalyzed Transformation of Organic Matter–Ferrihydrite Coprecipitates: A Closer Look Using Fe Isotopes
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-09-19 , DOI: 10.1021/acs.est.8b03407 Zhe Zhou 1 , Drew E. Latta 1 , Nadia Noor 2 , Aaron Thompson 2 , Thomas Borch 3, 4 , Michelle M. Scherer 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-09-19 , DOI: 10.1021/acs.est.8b03407 Zhe Zhou 1 , Drew E. Latta 1 , Nadia Noor 2 , Aaron Thompson 2 , Thomas Borch 3, 4 , Michelle M. Scherer 1
Affiliation
|
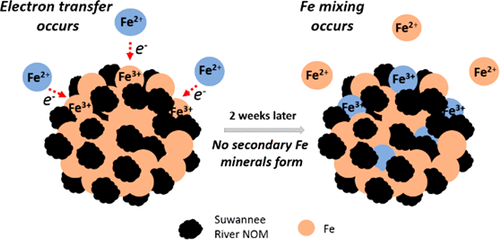
Ferrihydrite is a common Fe mineral in soils and sediments that rapidly transforms to secondary minerals in the presence of Fe(II). Both the rate and products of Fe(II)-catalyzed ferrihydrite transformation have been shown to be significantly influenced by natural organic matter (NOM). Here, we used enriched Fe isotope experiments and 57Fe Mössbauer spectroscopy to track the formation of secondary minerals, as well as electron transfer and Fe mixing between aqueous Fe(II) and ferrihydrite coprecipitated with several types of NOM. Ferrihydrite coprecipitated with humic acids transformed primarily to goethite after reaction with Fe(II). In contrast, ferrihydrite coprecipitated with fulvic acids and Suwannee River NOM (SRNOM) resulted in no measurable formation of secondary minerals. Despite no secondary mineral transformation, Mössbauer spectra indicated electron transfer still occurred between Fe(II) and ferrihydrite coprecipitated with fulvic acid and SRNOM. In addition, isotope tracer experiments revealed that a significant fraction of structural Fe in the ferrihydrite mixed with the aqueous phase Fe(II) (∼85%). After reaction with Fe(II), Mössbauer spectroscopy indicated some subtle changes in the crystallinity, particle size, or particle interactions in the coprecipitate. Our observations suggest that ferrihydrite coprecipitated with fulvic acid and SRNOM remains a highly dynamic phase even without ferrihydrite transformation.
中文翻译:

Fe(II)催化的有机物-水铁矿共沉淀物的转化:使用Fe同位素的近距离观察
水铁矿是土壤和沉积物中常见的铁矿物质,在存在Fe(II)的情况下会迅速转变为次生矿物质。Fe(II)催化的亚铁酸盐转变的速率和产物都显示出受天然有机物(NOM)的显着影响。在这里,我们使用了富铁同位素实验和57FeMössbauer光谱学可追踪次生矿物质的形成,以及在Fe(II)水溶液与三水合有几种NOM沉淀的亚铁酸盐之间的电子转移和Fe混合。与腐殖酸共沉淀的三水铁矿在与Fe(II)反应后主要转化为针铁矿。相反,水铁矿与富里酸和Suwannee River NOM(SRNOM)共沉淀导致没有可测量的次生矿物质形成。尽管没有二次矿物转化,但Mössbauer光谱表明,Fe(II)和与富里酸和SRNOM共沉淀的亚铁水合物之间仍发生电子转移。另外,同位素示踪实验表明,在三水铁矿中有很大一部分结构铁与水相Fe(II)混合(约85%)。与Fe(II)反应后,Mössbauer光谱表明,共沉淀物中的结晶度,粒径或粒子相互作用有一些细微的变化。我们的观察结果表明,即使没有亚铁酸盐转变,亚铁酸盐与富里酸和SRNOM一起共沉淀仍然是一个高度动态的相。
更新日期:2018-09-20
中文翻译:

Fe(II)催化的有机物-水铁矿共沉淀物的转化:使用Fe同位素的近距离观察
水铁矿是土壤和沉积物中常见的铁矿物质,在存在Fe(II)的情况下会迅速转变为次生矿物质。Fe(II)催化的亚铁酸盐转变的速率和产物都显示出受天然有机物(NOM)的显着影响。在这里,我们使用了富铁同位素实验和57FeMössbauer光谱学可追踪次生矿物质的形成,以及在Fe(II)水溶液与三水合有几种NOM沉淀的亚铁酸盐之间的电子转移和Fe混合。与腐殖酸共沉淀的三水铁矿在与Fe(II)反应后主要转化为针铁矿。相反,水铁矿与富里酸和Suwannee River NOM(SRNOM)共沉淀导致没有可测量的次生矿物质形成。尽管没有二次矿物转化,但Mössbauer光谱表明,Fe(II)和与富里酸和SRNOM共沉淀的亚铁水合物之间仍发生电子转移。另外,同位素示踪实验表明,在三水铁矿中有很大一部分结构铁与水相Fe(II)混合(约85%)。与Fe(II)反应后,Mössbauer光谱表明,共沉淀物中的结晶度,粒径或粒子相互作用有一些细微的变化。我们的观察结果表明,即使没有亚铁酸盐转变,亚铁酸盐与富里酸和SRNOM一起共沉淀仍然是一个高度动态的相。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号