Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Polyacrylamide Hydrogel Instability on High-Temperature Conditions
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-06 00:00:00 , DOI: 10.1021/acsomega.8b01205 Chunming Xiong 1 , Falin Wei 1 , Weitao Li 1 , Pingde Liu 1 , Yong Wu 2 , Mingli Dai 1 , Jun Chen 3
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-06 00:00:00 , DOI: 10.1021/acsomega.8b01205 Chunming Xiong 1 , Falin Wei 1 , Weitao Li 1 , Pingde Liu 1 , Yong Wu 2 , Mingli Dai 1 , Jun Chen 3
Affiliation
|
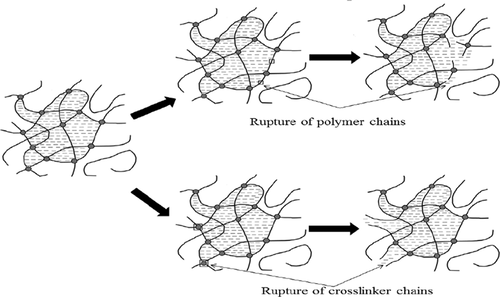
A gel system composed of acrylamide (AM), N,N′-methylenebisAM (BIS), and ammonium persulfate ((NH4)2S2O8) was developed and applied extensively in reservoirs to reduce water cut and increase oil production in mature fields. However, this gel system suffers from thermal stability loss and syneresis at high temperatures that reduces its ability to control water flow. It has been widely accepted that the loss of gel thermal stability can be explained via three aspects: the rupture of polymer chains, the breakage of cross-linker chains, and hydrolysis of polymer. The mechanism of hydrogel syneresis through polymer hydrolysis has been investigated extensively in other publications. However, research on the other two mechanisms is quite limited. In this article, we conduct a series of experiments to demonstrate how the rupture of polymer and cross-linker chains leads to the hydrogel instability at high temperatures. Viscosity and energy-dispersive system measurements suggested that polyAM chains were disrupted by the oxidation reactions involving free radicals. The method to measure the cross-linking degree was established and in combination with X-ray photoelectron spectroscopy measurements, the results showed that cross-linker chains were broken as a result of weaker C–N bond resulting from positively charged mesomethylene carbon and hydrolysis of amide groups on the cross-linker. Because of the application of deionized water in the experiments, nuclear magnetic resonance and FTIR measurements showed that the hydrolysis degree of polymer was weak. Hence, our results verified that breakage of polymer and cross-linker chains led to the rupture of the gel network at high temperature. Besides, cross-linker chains may play a more important role in the thermal stability of the gel, which explains some work into high-temperature-resistant gels.
中文翻译:

高温条件下聚丙烯酰胺水凝胶不稳定性的机理
由丙烯酰胺(AM),N,N'-亚甲基双AM (BIS)和过硫酸铵((NH 4)2 S 2 O 8)已开发并广泛用于储层,以减少成熟油田的含水量并提高石油产量。然而,这种凝胶体系在高温下遭受热稳定性损失和脱水收缩,从而降低了其控制水流的能力。凝胶热稳定性的丧失可以通过三个方面来解释:聚合物链的断裂,交联剂链的断裂以及聚合物的水解,这已被广泛接受。通过聚合物水解的水凝胶脱水收缩的机理已经在其他出版物中进行了广泛的研究。但是,对其他两种机制的研究非常有限。在本文中,我们进行了一系列实验,以证明聚合物和交联剂链的断裂如何导致高温下水凝胶的不稳定性。粘度和能量分散系统的测量表明,polyAM链被涉及自由基的氧化反应破坏。建立了测量交联度的方法,并与X射线光电子能谱法测量相结合,结果表明,交联剂链由于带正电的甲亚甲基碳水解和C-N键弱而导致C–N键弱而断裂。交联剂上的酰胺基。由于在实验中应用了去离子水,核磁共振和FTIR测量表明聚合物的水解度较弱。因此,我们的结果证实了聚合物和交联剂链的断裂导致高温下凝胶网络的断裂。除了,
更新日期:2018-09-06
中文翻译:

高温条件下聚丙烯酰胺水凝胶不稳定性的机理
由丙烯酰胺(AM),N,N'-亚甲基双AM (BIS)和过硫酸铵((NH 4)2 S 2 O 8)已开发并广泛用于储层,以减少成熟油田的含水量并提高石油产量。然而,这种凝胶体系在高温下遭受热稳定性损失和脱水收缩,从而降低了其控制水流的能力。凝胶热稳定性的丧失可以通过三个方面来解释:聚合物链的断裂,交联剂链的断裂以及聚合物的水解,这已被广泛接受。通过聚合物水解的水凝胶脱水收缩的机理已经在其他出版物中进行了广泛的研究。但是,对其他两种机制的研究非常有限。在本文中,我们进行了一系列实验,以证明聚合物和交联剂链的断裂如何导致高温下水凝胶的不稳定性。粘度和能量分散系统的测量表明,polyAM链被涉及自由基的氧化反应破坏。建立了测量交联度的方法,并与X射线光电子能谱法测量相结合,结果表明,交联剂链由于带正电的甲亚甲基碳水解和C-N键弱而导致C–N键弱而断裂。交联剂上的酰胺基。由于在实验中应用了去离子水,核磁共振和FTIR测量表明聚合物的水解度较弱。因此,我们的结果证实了聚合物和交联剂链的断裂导致高温下凝胶网络的断裂。除了,































 京公网安备 11010802027423号
京公网安备 11010802027423号