Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tetrapolymer Network Hydrogels via Gum Ghatti-Grafted and N-H/C-H-Activated Allocation of Monomers for Composition-Dependent Superadsorption of Metal Ions.
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-06 , DOI: 10.1021/acsomega.8b01218 Himarati Mondal 1 , Mrinmoy Karmakar 1 , Arnab Dutta 1 , Manas Mahapatra 1 , Mousumi Deb 1 , Madhushree Mitra 1 , Joy Sankar Deb Roy 1 , Chandan Roy 1 , Pijush Kanti Chattopadhyay 1 , Nayan Ranjan Singha 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-06 , DOI: 10.1021/acsomega.8b01218 Himarati Mondal 1 , Mrinmoy Karmakar 1 , Arnab Dutta 1 , Manas Mahapatra 1 , Mousumi Deb 1 , Madhushree Mitra 1 , Joy Sankar Deb Roy 1 , Chandan Roy 1 , Pijush Kanti Chattopadhyay 1 , Nayan Ranjan Singha 1
Affiliation
|
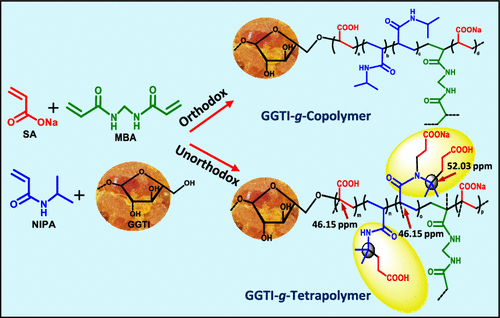
Herein, gum ghatti (GGTI)-g-[sodium acrylate (SA)-co-3-(N-(4-(4-methyl pentanoate))acrylamido)propanoate (NMPAP)-co-4-(acrylamido)-4-methyl pentanoate (AMP)-co-N-isopropylacrylamide (NIPA)] (i.e., GGTI-g-TetraP), a novel interpenetrating tetrapolymer network-based sustainable hydrogel, possessing extraordinary physicochemical properties and excellent recyclability, has been synthesized via grafting of GGTI and in situ strategic protrusion of NMPAP and AMP during the solution polymerization of SA and NIPA, through systematic multistage optimization of ingredients and temperature, for ligand-selective superadsorption of hazardous metal ions (M(II)), such as Sr(II), Hg(II), and Cu(II). The in situ allocation of NMPAP and AMP via N-H and C-H activations, grafting of GGTI into the SA-co-NMPAP-co-AMP-co-NIPA (TetraP) matrix, the effect of comonomer compositions on ligand-selective adsorption, crystallinity, thermal stabilities, surface properties, swellability, adsorption capacities (ACs), mechanical properties, and the superadsorption mechanism have been apprehended via extensive microstructural analyses of unloaded and/or loaded GGTI-g-TetraP1 and GGTI-g-TetraP2 bearing SA/NIPA in 8:1 and 2:1 ratios, respectively, using Fourier transform infrared (FTIR), 1H/13C/DEPT-135 NMR, X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis, differential scanning calorimetry, X-ray diffraction, field emission scanning electron microscopy, rheological analysis, and energy-dispersive X-ray spectrometry, along with measuring % gel content, pH at point of zero charge (pHPZC), and % graft ratio. The thermodynamically spontaneous chemisorption has been inferred from FTIR, XPS, fitting of kinetics data to pseudo-second-order model, and activation energies. The chemisorption data have exhibited excellent fitting to the Langmuir isotherm model. For Sr(II), Hg(II), and Cu(II), ACs were 1940.24/1748.36, 1759.50/1848.03, and 1903.64/1781.63 mg g-1, respectively, at 293 K, 0.02 g of GGTI-g-TetraP1/2, and initial concentration of M(II) = 500-1000 ppm.
中文翻译:

通过 Ghatti 接枝和 NH/CH 激活单体分配的四聚合物网络水凝胶,用于金属离子的成分依赖性超吸附。
本文中,加替胶(GGTI)-g-[丙烯酸钠(SA)-co-3-(N-(4-(4-戊酸甲酯))丙烯酰胺基)丙酸酯(NMPAP)-co-4-(丙烯酰胺基)-4 -戊酸甲酯(AMP)-共聚-N-异丙基丙烯酰胺(NIPA)](即GGTI-g-TetraP)是一种新型的基于互穿四聚物网络的可持续水凝胶,具有非凡的理化性能和优异的可回收性,通过接枝SA 和 NIPA 的溶液聚合过程中,GGTI 和 NMPAP 和 AMP 的原位战略突出,通过成分和温度的系统多级优化,用于有害金属离子 (M(II))(例如 Sr(II))的配体选择性超吸附、汞 (II) 和铜 (II)。通过 NH 和 CH 活化对 NMPAP 和 AMP 进行原位分配,将 GGTI 接枝到 SA-co-NMPAP-co-AMP-co-NIPA (TetraP) 基质中,共聚单体组成对配体选择性吸附、结晶度的影响,通过对未加载和/或加载的 GGTI-g-TetraP1 和 GGTI-g-TetraP2 轴承 SA/NIPA 进行广泛的微观结构分析,了解了热稳定性、表面性能、膨胀性、吸附能力 (AC)、机械性能和超吸附机制。分别使用傅里叶变换红外 (FTIR)、1H/13C/DEPT-135 NMR、X 射线光电子能谱 (XPS)、热重分析、差示扫描量热法、X 射线衍射、场分析,分别以 8:1 和 2:1 的比例进行分析发射扫描电子显微镜、流变分析和能量色散 X 射线光谱测定,以及测量凝胶含量百分比、零电荷点 pH (pHPZC) 和接枝率百分比。热力学自发化学吸附是通过 FTIR、XPS、动力学数据与伪二阶模型的拟合以及活化能推断出来的。化学吸附数据表现出与 Langmuir 等温线模型的良好拟合。对于 Sr(II)、Hg(II) 和 Cu(II),在 293 K、0.02 g GGTI-g-TetraP1 下,AC 分别为 1940.24/1748.36、1759.50/1848.03 和 1903.64/1781.63 mg g-1 /2,M(II) 的初始浓度 = 500-1000 ppm。
更新日期:2018-09-06
中文翻译:

通过 Ghatti 接枝和 NH/CH 激活单体分配的四聚合物网络水凝胶,用于金属离子的成分依赖性超吸附。
本文中,加替胶(GGTI)-g-[丙烯酸钠(SA)-co-3-(N-(4-(4-戊酸甲酯))丙烯酰胺基)丙酸酯(NMPAP)-co-4-(丙烯酰胺基)-4 -戊酸甲酯(AMP)-共聚-N-异丙基丙烯酰胺(NIPA)](即GGTI-g-TetraP)是一种新型的基于互穿四聚物网络的可持续水凝胶,具有非凡的理化性能和优异的可回收性,通过接枝SA 和 NIPA 的溶液聚合过程中,GGTI 和 NMPAP 和 AMP 的原位战略突出,通过成分和温度的系统多级优化,用于有害金属离子 (M(II))(例如 Sr(II))的配体选择性超吸附、汞 (II) 和铜 (II)。通过 NH 和 CH 活化对 NMPAP 和 AMP 进行原位分配,将 GGTI 接枝到 SA-co-NMPAP-co-AMP-co-NIPA (TetraP) 基质中,共聚单体组成对配体选择性吸附、结晶度的影响,通过对未加载和/或加载的 GGTI-g-TetraP1 和 GGTI-g-TetraP2 轴承 SA/NIPA 进行广泛的微观结构分析,了解了热稳定性、表面性能、膨胀性、吸附能力 (AC)、机械性能和超吸附机制。分别使用傅里叶变换红外 (FTIR)、1H/13C/DEPT-135 NMR、X 射线光电子能谱 (XPS)、热重分析、差示扫描量热法、X 射线衍射、场分析,分别以 8:1 和 2:1 的比例进行分析发射扫描电子显微镜、流变分析和能量色散 X 射线光谱测定,以及测量凝胶含量百分比、零电荷点 pH (pHPZC) 和接枝率百分比。热力学自发化学吸附是通过 FTIR、XPS、动力学数据与伪二阶模型的拟合以及活化能推断出来的。化学吸附数据表现出与 Langmuir 等温线模型的良好拟合。对于 Sr(II)、Hg(II) 和 Cu(II),在 293 K、0.02 g GGTI-g-TetraP1 下,AC 分别为 1940.24/1748.36、1759.50/1848.03 和 1903.64/1781.63 mg g-1 /2,M(II) 的初始浓度 = 500-1000 ppm。













































 京公网安备 11010802027423号
京公网安备 11010802027423号