European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-09-05 , DOI: 10.1016/j.ejmech.2018.08.094 Alessandro Giraudo , Jacob Krall , Birgitte Nielsen , Troels E. Sørensen , Kenneth T. Kongstad , Barbara Rolando , Donatella Boschi , Bente Frølund , Marco L. Lolli
|
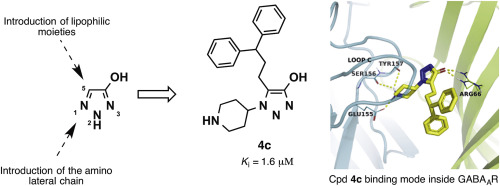
The correct application of bio(iso)steric replacement, a potent tool for the design of optimized compounds, requires the continuous development of new isosters able to respond to specific target requirements. Among carboxylic acid isosters, as the hydroxylated pentatomic heterocyclic systems, the hydroxy-1,2,3-triazole represents one of the most versatile but less investigated. With the purpose to enlarge its bioisosteric application, we report the results of a study devoted to obtain potential biomimetics of the γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system (CNS). A series of N1- and N2- functionalized 4-hydroxy-1,2,3-triazole analogues of the previous reported GABAAR ligands, including muscimol, 4-PIOL, and 4-PHP has been synthesized and characterized pharmacologically. Furthermore, this study led to development of straightforward chemical strategies directed to decorate the hydroxytriazole core scaffold, opening for further elaborative studies based on this system. The unsubstituted N1- and N2-piperidin-4-yl-4-hydroxy-1,2,3-triazole analogues (3a, 4a) of 4-PIOL and 4-PHP showed weak affinity (high to medium micromolar range), whereas substituting the 5-position of the triazole core with a 2-naphthylmethyl or 3,3-diphenylpropyl led to binding affinities in the low micromolar range. Based on electrostatic analysis and docking studies using a α1β2γ2 GABAAR homology model we were able to rationalize the observed divergence in SAR for the series of N1- and N2- piperidin-4-yl-4-hydroxy-1,2,3-triazole analogues, offering more detailed insight into the orthosteric GABAAR binding site.
中文翻译:

4-羟基-1,2,3-三唑部分作为羧酸的生物等排体:一种新型的支架,用于探测正构γ-氨基丁酸受体的结合位点
生物(等)位取代的正确应用(设计优化化合物的有效工具)需要不断开发能够响应特定目标要求的新等位基因。在羧酸的等排体中,作为羟基化的五原子杂环系统,羟基-1,2,3-三唑是用途最广但研究较少的一种。为了扩大其生物甾体的应用,我们报告了一项研究结果,该研究致力于获得γ-氨基丁酸(GABA)(中枢神经系统(CNS)中的主要抑制神经递质)的潜在仿生体。一系列的N 1-和N 2-已经合成并在药理学上表征了先前报道的GABA A R配体的功能化的4-羟基-1,2,3-三唑类似物,包括muscimol,4-PIOL和4-PHP。此外,这项研究还导致了直接化学方法的发展,该策略旨在装饰羟基三唑核心支架,为基于该系统的进一步详细研究打开了方便之门。未取代的N 1-和N 2-哌啶-4-基-4-羟基-1,2,3-三唑类似物(3a,4a)的4-PIOL和4-PHP表现出较弱的亲和力(高至中微摩尔范围),而用2-萘甲基或3,3-二苯丙基取代三唑核心的5位导致在低微摩尔范围内的结合亲和力。基于静电分析和使用α对接研究1 β 2 γ 2 GABA甲ř同源性模型,我们能够合理化观察到的差异在SAR为系列Ñ 1 -和ñ 2 -哌啶-4-基-4-羟基-1,2,3-三唑类似物,可更深入地了解正构GABA A R结合位点。































 京公网安备 11010802027423号
京公网安备 11010802027423号