当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural, Mechanistic and Ultra-dilute Catalysis Portrayal of Substrate Inhibition in the TAML–Hydrogen Peroxide Catalytic Oxidation of the Persistent Drug and Micropollutant, Propranolol
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-05 , DOI: 10.1021/jacs.8b08108 Yogesh Somasundar 1 , Longzhu Q. Shen 2 , Alexis G. Hoane 1 , Liang L. Tang 1 , Matthew R. Mills 1 , Abigail E. Burton 1 , Alexander D. Ryabov 1 , Terrence J. Collins 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-05 , DOI: 10.1021/jacs.8b08108 Yogesh Somasundar 1 , Longzhu Q. Shen 2 , Alexis G. Hoane 1 , Liang L. Tang 1 , Matthew R. Mills 1 , Abigail E. Burton 1 , Alexander D. Ryabov 1 , Terrence J. Collins 1
Affiliation
|
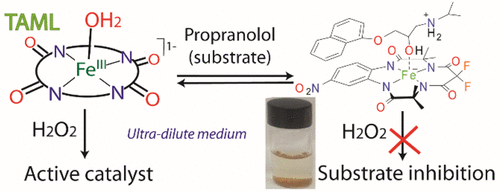
TAML activators enable unprecedented, rapid, ultradilute oxidation catalysis where substrate inhibitions might seem improbable. Nevertheless, while TAML/H2O2 rapidly degrades the drug propranolol, a micropollutant (MP) of broad concern, propranolol is shown to inhibit its own destruction under concentration conditions amenable to kinetics studies ([propranolol] = 50 μM). Substrate inhibition manifests as a decrease in the second-order rate constant kI for H2O2 oxidation of the resting FeIII-TAML (RC) to the activated catalyst (AC), while the second-order rate constant kII for attack of AC on propranolol is unaffected. This kinetics signature has been utilized to develop a general approach for quantifying substrate inhibitions. Fragile adducts [propranolol, TAML] have been isolated and subjected to ESI-MS, florescence, UV-vis, FTIR, 1H NMR, and IC examination and DFT calculations. Propranolol binds to FeIII-TAMLs via combinations of noncovalent hydrophobic, coordinative, hydrogen bonding, and Coulombic interactions. Across four studied TAMLs under like conditions, propranolol reduced kI 4-32-fold (pH 7, 25 °C) indicating that substrate inhibition is controllable by TAML design. However, based on the measured kI and calculated equilibrium constant K for propranolol-TAML binding, it is possible to project the impact on kI of reducing [propranolol] from 50 μM to the ultradilute regime typical of MP contaminated waters (≤2 ppb, ≤7 nM for propranolol) where inhibition nearly vanishes. Projecting from 50 μM to higher concentrations, propranolol completely inhibits its own oxidation before reaching mM concentrations. This study is consistent with prior experimental findings that substrate inhibition does not impede TAML/H2O2 destruction of propranolol in London wastewater while giving a substrate inhibition assessment tool for use in the new field of ultradilute oxidation catalysis.
中文翻译:

TAML-过氧化氢催化氧化持久性药物和微污染物普萘洛尔中底物抑制的结构、机理和超稀催化描述
TAML 激活剂可实现前所未有的、快速的、超稀释的氧化催化,其中底物抑制似乎不太可能。然而,虽然 TAML/H2O2 会迅速降解药物普萘洛尔,这是一种受到广泛关注的微量污染物 (MP),但研究表明,普萘洛尔在适合动力学研究的浓度条件下([普萘洛尔] = 50 μM)可抑制其自身的破坏。底物抑制表现为 H2O2 将静止 FeIII-TAML (RC) 氧化为活化催化剂 (AC) 的二级速率常数 kI 降低,而 AC 对普萘洛尔的攻击的二级速率常数 kII 不受影响. 该动力学特征已被用于开发量化底物抑制的通用方法。脆性加合物 [心得安、TAML] 已被分离出来并进行了 ESI-MS、荧光、紫外-可见光、FTIR、1H NMR、和IC检查和DFT计算。普萘洛尔通过非共价疏水、配位、氢键和库仑相互作用的组合与 FeIII-TAML 结合。在类似条件下研究的四个 TAML 中,普萘洛尔将 kI 降低了 4-32 倍(pH 7,25°C),表明底物抑制可通过 TAML 设计进行控制。然而,根据普萘洛尔-TAML 结合的测量 kI 和计算平衡常数 K,可以预测将 [普萘洛尔] 从 50 μM 降低到典型的 MP 污染水体(≤2 ppb,≤ 7 nM(心得安),抑制几乎消失。从 50 μM 投射到更高浓度,普萘洛尔在达到 mM 浓度之前完全抑制其自身的氧化。
更新日期:2018-09-05
中文翻译:

TAML-过氧化氢催化氧化持久性药物和微污染物普萘洛尔中底物抑制的结构、机理和超稀催化描述
TAML 激活剂可实现前所未有的、快速的、超稀释的氧化催化,其中底物抑制似乎不太可能。然而,虽然 TAML/H2O2 会迅速降解药物普萘洛尔,这是一种受到广泛关注的微量污染物 (MP),但研究表明,普萘洛尔在适合动力学研究的浓度条件下([普萘洛尔] = 50 μM)可抑制其自身的破坏。底物抑制表现为 H2O2 将静止 FeIII-TAML (RC) 氧化为活化催化剂 (AC) 的二级速率常数 kI 降低,而 AC 对普萘洛尔的攻击的二级速率常数 kII 不受影响. 该动力学特征已被用于开发量化底物抑制的通用方法。脆性加合物 [心得安、TAML] 已被分离出来并进行了 ESI-MS、荧光、紫外-可见光、FTIR、1H NMR、和IC检查和DFT计算。普萘洛尔通过非共价疏水、配位、氢键和库仑相互作用的组合与 FeIII-TAML 结合。在类似条件下研究的四个 TAML 中,普萘洛尔将 kI 降低了 4-32 倍(pH 7,25°C),表明底物抑制可通过 TAML 设计进行控制。然而,根据普萘洛尔-TAML 结合的测量 kI 和计算平衡常数 K,可以预测将 [普萘洛尔] 从 50 μM 降低到典型的 MP 污染水体(≤2 ppb,≤ 7 nM(心得安),抑制几乎消失。从 50 μM 投射到更高浓度,普萘洛尔在达到 mM 浓度之前完全抑制其自身的氧化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号