当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anhydrous Methanol and Ethanol Dehydrogenation at Cu(111) Step Edges
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-09-18 , DOI: 10.1021/acs.jpcc.8b06730 Robert A. Hoyt , Matthew M. Montemore , E. Charles H. Sykes 1 , Efthimios Kaxiras
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-09-18 , DOI: 10.1021/acs.jpcc.8b06730 Robert A. Hoyt , Matthew M. Montemore , E. Charles H. Sykes 1 , Efthimios Kaxiras
Affiliation
|
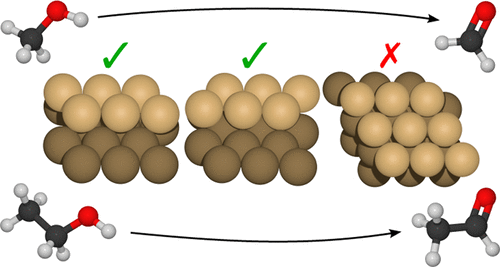
Oxidative methanol dehydrogenation is a major industrial reaction with global formaldehyde production exceeding 30 million tonnes per year. Unfortunately, oxidative dehydrogenation produces water–aldehyde mixtures that require subsequent distillation. Anhydrous alcohol dehydrogenation is a promising alternative that produces H2 instead of water. Pursuant to recent experimental work showing that highly stepped Cu(111) surfaces exhibit anhydrous dehydrogenation activity, we present first-principles density functional theory calculations for methanol and ethanol dehydrogenation at Cu(111) step edges to provide an atomistic understanding of the catalytic mechanism; these sites stabilize all intermediates while reducing activation energies. We find that van der Waals contributions to the energy account for more than 50% of adsorption energies, and their inclusion is essential in achieving good agreement with experimental desorption temperatures. Furthermore, vibrational zero-point energy corrections significantly reduce the activation energy for all reaction steps considered here. Hydrogen bonding among ethanol intermediates at step edges is weakened by geometric frustration. These insights lead us to propose several suggestions for further research on undercoordinated Cu sites as anhydrous alcohol dehydrogenation catalysts.
中文翻译:

Cu(111)台阶边缘的无水甲醇和乙醇脱氢
氧化甲醇脱氢是一项重大的工业反应,全球甲醛年产量超过3000万吨。不幸的是,氧化脱氢产生的水-醛混合物需要随后的蒸馏。无水乙醇脱氢是产生H 2的有前途的替代方法而不是水。根据最近的实验工作表明高度阶梯化的Cu(111)表面表现出无水脱氢活性,我们提出了在Cu(111)台阶边缘处甲醇和乙醇脱氢的第一原理密度泛函理论计算,以提供对催化机理的原子学理解;这些位点稳定所有中间体,同时降低活化能。我们发现范德华对能量的贡献占吸附能的50%以上,并且它们的包含对于与实验解吸温度达成良好一致性至关重要。此外,振动零点能量校正显着降低了此处考虑的所有反应步骤的活化能。台阶处的乙醇中间体之间的氢键会因几何受挫而减弱。
更新日期:2018-09-19
中文翻译:

Cu(111)台阶边缘的无水甲醇和乙醇脱氢
氧化甲醇脱氢是一项重大的工业反应,全球甲醛年产量超过3000万吨。不幸的是,氧化脱氢产生的水-醛混合物需要随后的蒸馏。无水乙醇脱氢是产生H 2的有前途的替代方法而不是水。根据最近的实验工作表明高度阶梯化的Cu(111)表面表现出无水脱氢活性,我们提出了在Cu(111)台阶边缘处甲醇和乙醇脱氢的第一原理密度泛函理论计算,以提供对催化机理的原子学理解;这些位点稳定所有中间体,同时降低活化能。我们发现范德华对能量的贡献占吸附能的50%以上,并且它们的包含对于与实验解吸温度达成良好一致性至关重要。此外,振动零点能量校正显着降低了此处考虑的所有反应步骤的活化能。台阶处的乙醇中间体之间的氢键会因几何受挫而减弱。













































 京公网安备 11010802027423号
京公网安备 11010802027423号