当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Study on Magnesium Ion Solvation in Diglyme-Based Electrolytes: IR Spectroscopy and DFT Calculations
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-09-11 , DOI: 10.1021/acs.jpcb.8b05586 Kenta Fujii 1 , Michiru Sogawa 1 , Nobuko Yoshimoto 1 , Masayuki Morita 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-09-11 , DOI: 10.1021/acs.jpcb.8b05586 Kenta Fujii 1 , Michiru Sogawa 1 , Nobuko Yoshimoto 1 , Masayuki Morita 1
Affiliation
|
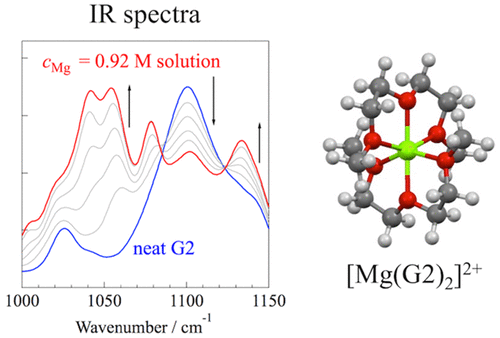
We investigated the solvation structure of Mg ions in a diglyme (G2)-based electrolyte solution for Mg ion batteries. The Walden plots based on ionic conductivity and viscosity of the Mg(TFSA)2/G2 [TFSA: bis(trifluoromethanesulfonyl)amide] solutions indicated that the dissociativity of Mg(TFSA)2 gradually increased, even with increasing salt concentration (cMg). This behavior is similar to that of the analogous triglyme (G3)-based solutions. Infrared (IR) spectroscopy revealed that Mg ions were coordinated by two G2 molecules to form an octahedral [Mg(G2)2]2+ complex in the cMg range examined herein (≤0.92 M). The detailed coordination geometry of the [Mg(G2)2]2+ complex was evaluated using density functional theory calculations. We found that G2 molecules coordinated in a tridentate ligand fashion to form an octahedral [Mg(tri-G2)2]2+ complex. This result was different from that of the G3 system; i.e., G3 molecules acted in three ligand modes (bidentate, tridentate, and tetradentate) such that multiple solvation complexes such as [Mg(tri-G3)2]2+ and [Mg(bi-G3)(tetra-G3)]2+ complexes were formed. This difference between the G2 and G3 systems might be related to an entropy contribution in the liquid state; i.e., only one coordination structure exists for [Mg(tri-G2)2]2+ in the G2 system, whereas more coordination complex structures can be formed in the G3 system.
中文翻译:

基于二甘醇二甲醚的电解质中镁离子溶剂化的结构研究:红外光谱和DFT计算
我们研究了镁离子电池中基于二甘醇二甲醚(G2)的电解质溶液中镁离子的溶剂化结构。基于Mg(TFSA)2 / G2 [TFSA:双(三氟甲磺酰基)酰胺]溶液的离子电导率和粘度的Walden图表明,即使盐浓度(c Mg)增加,Mg(TFSA)2的离解性也逐渐增加。。此行为类似于类似的基于三字符(G3)的解决方案。红外光谱显示,Mg离子由两个G2分子配位,在c Mg中形成八面体[Mg(G2)2 ] 2+络合物此处检查的范围(≤0.92M)。[Mg(G2)2 ] 2+配合物的详细配位几何结构使用密度泛函理论计算进行了评估。我们发现,G2分子以三齿配体的方式协调形成八面体[Mg(tri-G2)2 ] 2+络合物。此结果与G3系统的结果不同;即,G3分子以三种配体模式(双齿,三齿和四齿)起作用,从而形成多个溶剂化复合物,例如[Mg(tri-G3)2 ] 2+和[Mg(bi-G3)(tetra-G3)] 2 +复合物形成。G2和G3系统之间的这种差异可能与液态中的熵贡献有关。也就是说,在G2系统中[Mg(tri-G2)2 ] 2+仅存在一种配位结构,而在G3系统中可以形成更多的配位复杂结构。
更新日期:2018-09-11
中文翻译:

基于二甘醇二甲醚的电解质中镁离子溶剂化的结构研究:红外光谱和DFT计算
我们研究了镁离子电池中基于二甘醇二甲醚(G2)的电解质溶液中镁离子的溶剂化结构。基于Mg(TFSA)2 / G2 [TFSA:双(三氟甲磺酰基)酰胺]溶液的离子电导率和粘度的Walden图表明,即使盐浓度(c Mg)增加,Mg(TFSA)2的离解性也逐渐增加。。此行为类似于类似的基于三字符(G3)的解决方案。红外光谱显示,Mg离子由两个G2分子配位,在c Mg中形成八面体[Mg(G2)2 ] 2+络合物此处检查的范围(≤0.92M)。[Mg(G2)2 ] 2+配合物的详细配位几何结构使用密度泛函理论计算进行了评估。我们发现,G2分子以三齿配体的方式协调形成八面体[Mg(tri-G2)2 ] 2+络合物。此结果与G3系统的结果不同;即,G3分子以三种配体模式(双齿,三齿和四齿)起作用,从而形成多个溶剂化复合物,例如[Mg(tri-G3)2 ] 2+和[Mg(bi-G3)(tetra-G3)] 2 +复合物形成。G2和G3系统之间的这种差异可能与液态中的熵贡献有关。也就是说,在G2系统中[Mg(tri-G2)2 ] 2+仅存在一种配位结构,而在G3系统中可以形成更多的配位复杂结构。













































 京公网安备 11010802027423号
京公网安备 11010802027423号