当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stoichiometric network analysis of entropy production in chemical reactions
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-09-03 00:00:00 , DOI: 10.1039/c8cp04398a David Hochberg 1, 2, 3, 4 , Josep M. Ribó 4, 5, 6, 7, 8
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-09-03 00:00:00 , DOI: 10.1039/c8cp04398a David Hochberg 1, 2, 3, 4 , Josep M. Ribó 4, 5, 6, 7, 8
Affiliation
|
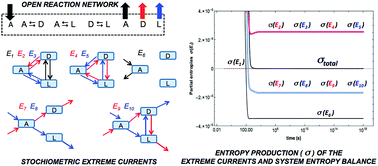
We use stoichiometric network analysis (SNA) to obtain a flux-based approach for the evaluation and description of the entropy production and exchange for chemical reactions in open systems. For non-equilibrium stationary states (NESS) the production and exchange are expressed as functions over the convex cone of the stationary reaction rates, revealing the reaction pathways and elementary flux modes (EFM) responsible for both entropy production and balance. The analysis of the entropy production of EFMs leads to a unique description of the contribution of the coupling between linear or cyclic reaction network paths, and the fluxes due to the matter exchange of the system with the system's surroundings. Network stoichiometry leads to an independent proof and confirmation of Prigogine's theorem of minimum entropy production for the linear regime of non-equilibrium thermodynamics. Moreover, the non-linear thermodynamic regime allows us to test the validity of the General Evolution Criterion (GEC) for NESS in isothermal chemical networks in mechanical equilibrium.
中文翻译:

化学反应中熵产生的化学计量网络分析
我们使用化学计量网络分析(SNA)获得基于通量的方法,用于评估和描述开放系统中化学反应的熵产和交换。对于非平衡稳态(NESS),生成和交换表示为稳态反应速率凸锥上的函数,从而揭示了负责熵生成和平衡的反应途径和基本通量模式(EFM)。对EFM的熵产生的分析导致对线性或循环反应网络路径之间的耦合的贡献以及由于系统与系统环境之间的物质交换而导致的通量的独特描述。网络化学计量学导致对Prigogine'的独立证明和确认 非平衡热力学线性系统的最小熵定理。此外,非线性热力学机制使我们能够在机械平衡的等温化学网络中测试NESS的通用演化准则(GEC)的有效性。
更新日期:2018-09-03
中文翻译:

化学反应中熵产生的化学计量网络分析
我们使用化学计量网络分析(SNA)获得基于通量的方法,用于评估和描述开放系统中化学反应的熵产和交换。对于非平衡稳态(NESS),生成和交换表示为稳态反应速率凸锥上的函数,从而揭示了负责熵生成和平衡的反应途径和基本通量模式(EFM)。对EFM的熵产生的分析导致对线性或循环反应网络路径之间的耦合的贡献以及由于系统与系统环境之间的物质交换而导致的通量的独特描述。网络化学计量学导致对Prigogine'的独立证明和确认 非平衡热力学线性系统的最小熵定理。此外,非线性热力学机制使我们能够在机械平衡的等温化学网络中测试NESS的通用演化准则(GEC)的有效性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号