EMBO Reports ( IF 6.5 ) Pub Date : 2018-10-01 , DOI: 10.15252/embr.201744445
Marek Kozlowski 1 , David Corujo 2, 3 , Michael Hothorn 4 , Iva Guberovic 2 , Imke K Mandemaker 1 , Charlotte Blessing 1 , Judith Sporn 4 , Arturo Gutierrez‐Triana 4 , Rebecca Smith 1 , Thomas Portmann 4 , Mathias Treier 4 , Klaus Scheffzek 4 , Sebastien Huet 5 , Gyula Timinszky 1 , Marcus Buschbeck 2, 6 , Andreas G Ladurner 1, 7, 8
|
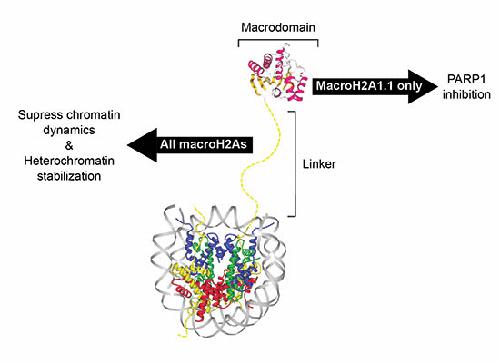
MacroH2A histone variants suppress tumor progression and act as epigenetic barriers to induced pluripotency. How they impart their influence on chromatin plasticity is not well understood. Here, we analyze how the different domains of macroH2A proteins contribute to chromatin structure and dynamics. By solving the crystal structure of the macrodomain of human macroH2A2 at 1.7 Å, we find that its putative binding pocket exhibits marked structural differences compared with the macroH2A1.1 isoform, rendering macroH2A2 unable to bind ADP‐ribose. Quantitative binding assays show that this specificity is conserved among vertebrate macroH2A isoforms. We further find that macroH2A histones reduce the transient, PARP1‐dependent chromatin relaxation that occurs in living cells upon DNA damage through two distinct mechanisms. First, macroH2A1.1 mediates an isoform‐specific effect through its ability to suppress PARP1 activity. Second, the unstructured linker region exerts an additional repressive effect that is common to all macroH2A proteins. In the absence of DNA damage, the macroH2A linker is also sufficient for rescuing heterochromatin architecture in cells deficient for macroH2A.
中文翻译:

MacroH2A组蛋白变异体通过两种不同的机制限制了染色质的可塑性
MacroH2A组蛋白变异体可抑制肿瘤进展并充当诱导多能性的表观遗传屏障。他们如何赋予他们对染色质可塑性的影响尚不清楚。在这里,我们分析了macroH2A蛋白的不同结构域如何促进染色质结构和动力学。通过在1.7Å处解析人macroH2A2宏域的晶体结构,我们发现其假定的结合口袋与macroH2A1.1同工型相比具有明显的结构差异,从而使macroH2A2无法结合ADP核糖。定量结合试验表明,这种特异性在脊椎动物macroH2A同工型中是保守的。我们进一步发现macroH2A组蛋白降低了在活细胞中发生的瞬时PARP 1依赖性染色质松弛DNA的损伤通过两种不同的机制。首先,macroH2A1.1通过抑制PARP 1的活性介导同种型特异的作用。第二,非结构化的接头区域发挥了所有macroH2A蛋白共有的额外抑制作用。在没有DNA损伤的情况下,macroH2A接头也足以拯救缺乏macroH2A的细胞中的异染色质结构。

































 京公网安备 11010802027423号
京公网安备 11010802027423号