Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2018-09-01 , DOI: 10.1016/j.cej.2018.08.224 Long Zhang , Fenglian Fu , Bing Tang
|
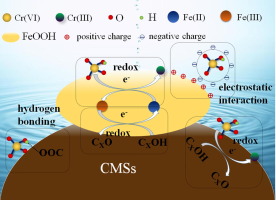
In this study, novel mesoporous composites of goethite/carbon microspheres (α-FeOOH/CMSs) and akaganeite/carbon microspheres (β-FeOOH/CMSs) were successfully prepared and employed to remove Cr(VI). Scanning electron microscopy and transmission electron microscopy of α-FeOOH/CMSs and β-FeOOH/CMSs composites indicated α-FeOOH and β-FeOOH crystals were dispersed on the surface of CMSs. From Brunauer–Emmett–Teller surface area measurement, the average pore widths of α-FeOOH/CMSs and β-FeOOH/CMSs composites were 6.1 and 2.4 nm, respectively, indicating that α-FeOOH/CMSs and β-FeOOH/CMSs were mesoporous materials. The results of batch experiments showed that the performance of α-FeOOH/CMSs in Cr(VI) removal was better than β-FeOOH/CMSs. The removal efficiencies of Cr(VI) by α-FeOOH/CMSs were all over 90.0% after 180 min in the pH range from 3.0 to 9.0. The adsorption of Cr(VI) onto α-FeOOH/CMSs and β-FeOOH/CMSs composites followed the Freundlich isotherm model and pseudo-second-order kinetic model. The maximum adsorption capacities of Cr(VI) by α-FeOOH/CMSs and β-FeOOH/CMSs composites are 55.37 and 29.37 mg/g, respectively. The coexisting Cl−, Cu2+, and Ni2+ had no significant effect on Cr(VI) removal, whereas SO42− and PO43− hindered the removal of Cr(VI). Through X–ray photoelectron spectroscopy, Cr(VI) can not only be directly adsorbed on α-FeOOH/CMSs and β-FeOOH/CMSs composites, but also be reduced to less toxic Cr(III). The α-FeOOH/CMSs exhibited great potential on the removal of Cr(VI) from wastewater.
中文翻译:

针铁矿/碳微球和赤铁矿/碳微球复合材料对Cr(VI)的吸附和氧化还原行为
在这项研究中,成功地制备了针铁矿/碳微球(α-FeOOH/ CMSs)和赤铁矿/碳微球(β-FeOOH/ CMSs)的新型介孔复合材料,并用于去除Cr(VI)。α-FeOOH/ CMSs和β-FeOOH/ CMSs复合材料的扫描电子显微镜和透射电镜表明,α-FeOOH和β-FeOOH晶体分散在CMSs表面。根据Brunauer-Emmett-Teller表面积测量,α-FeOOH/ CMSs和β-FeOOH/ CMSs复合材料的平均孔径分别为6.1和2.4 nm,表明α-FeOOH/ CMSs和β-FeOOH/ CMSs是中孔的材料。批处理实验结果表明,α-FeOOH/ CMSs去除Cr(VI)的性能优于β-FeOOH/ CMSs。在3.0至9.0的pH范围内,180分钟后α-FeOOH / CMS对Cr(VI)的去除率均超过90.0%。Cr(VI)在α-FeOOH/ CMS和β-FeOOH/ CMSs复合材料上的吸附遵循Freundlich等温模型和伪二级动力学模型。α-FeOOH/ CMSs和β-FeOOH/ CMSs复合材料对Cr(VI)的最大吸附容量分别为55.37和29.37 mg / g。共存的Cl-,Cu 2+和Ni 2+对Cr(VI)的去除没有显着影响,而SO 4 2-和PO 4 3-则阻碍了Cr(VI)的去除。通过X射线光电子能谱,不仅可以将Cr(VI)直接吸附在α-FeOOH/ CMS和β-FeOOH/ CMSs复合材料上,还可以将其还原为毒性较小的Cr(III)。α-FeOOH/ CMS在去除废水中的Cr(VI)方面显示出巨大的潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号