Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2018-09-01 , DOI: 10.1016/j.cej.2018.08.225 Xi Liu , Shaojian Jiang , Hailong Li , Jianping Yang , Zequn Yang , Jiexia Zhao , Haoyi Peng , Kaimin Shih
|
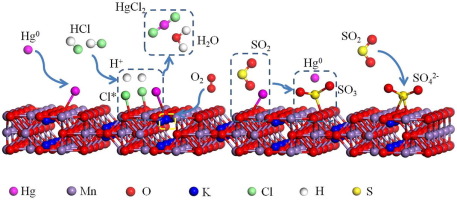
Manganese oxide octahedral molecular sieve (OMS-2) with cryptomelane structure synthesized by a solvent-free method was employed to oxidize gaseous elemental mercury (Hg0) in coal combustion flue gas for the first time. Brunauer-Emmett-Teller (BET) surface area analysis, X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectra, H2 temperature-programmed reduction (H2-TPR) and X-ray photoelectron spectroscopy (XPS) measurement were employed to characterize the catalyst. The OMS-2 with large surface area and abundant pores presented excellent Hg0 oxidation performance at a wide temperature range of 100–250 °C. At the optimal operating temperature of 150 °C, 93% Hg0 oxidation efficiency was obtained under a gas hourly space velocity as high as 1,350,000 h−1. Hg0 oxidation on OMS-2 catalyst was supposed to follow the Mars-Maessen mechanism, where the gaseous Hg0 was firstly adsorbed on the OMS-2 catalyst surface to form adsorbed Hg0, and then the adsorbed Hg0 was oxidized by the lattice oxygen over catalyst surface to form mercury oxide (HgO). Both HCl and NO in the flue gas promoted Hg0 oxidation mainly due to the formation of active species like Cl∗, NO2, which reacted with Hg0 to form volatile mercury species. SO2 inhibited the Hg0 oxidation by reducing the adsorbed HgO into gaseous Hg0 or generating sulfate on the catalyst surface. Water vapor also played an inhibitive role in Hg0 oxidation. However, the SO2 and H2O resistance of OMS-2 catalyst was much superior compared to other commercial catalysts, which makes it promising for industrial application. This knowledge is beneficial for developing economical and efficient Hg0 oxidation catalysts for coal-fired power plants.
中文翻译:

烟气温度低下氧化锰八面体分子筛催化剂上的元素汞氧化
采用无溶剂法合成的具有隐甲烷结构的氧化锰八面体分子筛(OMS-2)首次用于氧化煤燃烧烟气中的气态元素汞(Hg 0)。Brunauer-Emmett-Teller(BET)表面积分析,X射线衍射(XRD),傅立叶变换红外(FT-IR)光谱,H 2程序升温还原(H 2 -TPR)和X射线光电子能谱(XPS) )测量用于表征催化剂。具有大表面积和丰富孔隙的OMS-2在100–250°C的宽温度范围内均具有出色的Hg 0氧化性能。在150°C的最佳工作温度下,93%Hg 0在高达每小时1,350,000 h -1的气体时空速下获得氧化效率。假设OMS-2催化剂上的Hg 0氧化遵循Mars-Maessen机理,其中气态Hg 0首先被吸附在OMS-2催化剂表面上,形成吸附的Hg 0,然后吸附的Hg 0被晶格氧化。氧气在催化剂表面上形成氧化汞(HgO)。烟道气中的HCl和NO都促进了Hg 0的氧化,这主要是由于形成了诸如Cl ∗,NO 2之类的活性物质,它们与Hg 0反应形成了挥发性的汞物质。SO 2抑制Hg 0通过将吸附的HgO还原为气态Hg 0或在催化剂表面生成硫酸盐来进行氧化。水蒸气在Hg 0氧化中也起抑制作用。但是,OMS-2催化剂的耐SO 2和H 2 O性能远远优于其他商用催化剂,这使其在工业上具有广阔的应用前景。该知识对于开发用于燃煤电厂的经济有效的Hg 0氧化催化剂是有益的。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号