Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discrete Existence of Singlet Nitrenium Ions Revisited: Computational Studies of Non-Aryl Nitrenium Ions and Their Rearrangements
ACS Omega ( IF 3.7 ) Pub Date : 2018-08-31 00:00:00 , DOI: 10.1021/acsomega.8b01038 Daniel E Falvey 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-08-31 00:00:00 , DOI: 10.1021/acsomega.8b01038 Daniel E Falvey 1
Affiliation
|
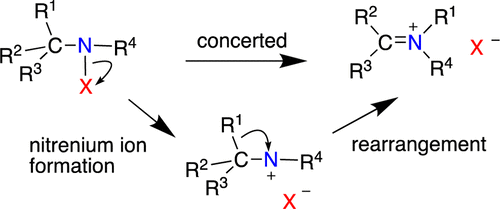
Nitrenium ion species are examined using computational methods (DFT, MP2, coupled-cluster, and a composite method, CBS-APNO) with a particular emphasis on nonaromatic species (i.e., those lacking an aromatic or heteroaromatic ring in direct conjugation with the formal nitrenium ion center.) The substitution of the N+ center with alkyl, alkoxy, vinyl, acyl, and sulfonyl, among others, was evaluated. For these species, three properties are considered. (1) The stability of the nitrenium ions to unimolecular isomerizations such as 1,2 alkyl or H shifts; to the extent that the singlet states could be characterized as discrete minima on the potential energy surface (PES), (2) the effect of the substituents on singlet–triplet energy splitting as well as (3) the relative stabilities of the nitrenium ions as defined by N-hydration enthalpies (RR′N+ + H2O → RR′NOH2+). Nearly all simple alkyl and di-alkyl nitrenium ion singlet states are predicted to rearrange without detectable barriers, largely through 1,2 H or alkyl shifts. Methyl and N,N-dimethylnitrenium ion singlet states could be characterized as formal minima on the PES. However, these species show small or insignificant barriers to isomerization. Disubstituted nitrenium ions that include an alkyl group and a conjugating substituent such as alkoxyl, vinyl, or phenyl show meaningful barriers to isomerization and are thus predicted to possess nontrivial lifetimes in solution. Alkyl groups substantially stabilize the singlet state relative to the situation in the parent nitrenium ion NH2+ to the point where the two states are nearly degenerate. Other groups that interact with the nitrenium ion center decrease the ΔEst in the order formoyl < vinyl < phenyl < alkoxy ∼ sulfonyl < cyclopropyl ∼ cyclobutyl. The latter two substituents interact strongly with the (singlet) nitrenium ion center through the formation of a nonclassical bonding reminiscent of the bisected cyclopropylcarbinyl ion case for carbocations. When singlet-state stability is evaluated in the context of N-hydration enthalpies, it is found that the ordering is acyl < vinyl < alkoxyl < phenyl < cyclopropyl and cyclobutyl.
中文翻译:

重新审视单线态氮离子的离散存在:非芳基氮离子及其重排的计算研究
使用计算方法(DFT、MP2、耦合簇和复合方法,CBS-APNO)检查氮离子物质,特别强调非芳香族物质(即那些缺乏与正式氮直接共轭的芳香族或杂芳香环的物质)离子中心。)评估了 N +中心被烷基、烷氧基、乙烯基、酰基和磺酰基等取代。对于这些物种,需要考虑三个属性。(1)氮离子对单分子异构化如1,2烷基或H位移的稳定性;在单线态可以表征为势能面(PES)上的离散最小值的范围内,(2)取代基对单线态-三线态能量分裂的影响以及(3)氮离子的相对稳定性由 N-水合焓 (RR′N + + H 2 O → RR′NOH 2 + ) 定义。预计几乎所有简单的烷基和二烷基氮鎓离子单线态都会在没有可检测障碍的情况下重新排列,主要是通过 1,2 H 或烷基位移。甲基和N , N-二甲基氮鎓离子单线态可以表征为 PES 上的形式最小值。然而,这些物质对异构化表现出很小或不显着的障碍。包含烷基和共轭取代基(例如烷氧基、乙烯基或苯基)的二取代氮鎓离子对异构化表现出有意义的障碍,因此预计在溶液中具有非凡的寿命。相对于母体氮离子NH 2 +的情况,烷基基本上将单线态稳定到两种状态几乎简并的程度。其他与氮离子中心相互作用的基团按甲酰基 < 乙烯基 < 苯基 < 烷氧基 ~ 磺酰基 < 环丙基 ~ 环丁基的顺序降低 Δ E st 。后两个取代基通过形成非经典键与(单态)氮离子中心强烈相互作用,让人想起碳阳离子的平分环丙基碳酰离子情况。当在N-水合焓的背景下评估单线态稳定性时,发现排序为酰基<乙烯基<烷氧基<苯基<环丙基和环丁基。
更新日期:2018-08-31
中文翻译:

重新审视单线态氮离子的离散存在:非芳基氮离子及其重排的计算研究
使用计算方法(DFT、MP2、耦合簇和复合方法,CBS-APNO)检查氮离子物质,特别强调非芳香族物质(即那些缺乏与正式氮直接共轭的芳香族或杂芳香环的物质)离子中心。)评估了 N +中心被烷基、烷氧基、乙烯基、酰基和磺酰基等取代。对于这些物种,需要考虑三个属性。(1)氮离子对单分子异构化如1,2烷基或H位移的稳定性;在单线态可以表征为势能面(PES)上的离散最小值的范围内,(2)取代基对单线态-三线态能量分裂的影响以及(3)氮离子的相对稳定性由 N-水合焓 (RR′N + + H 2 O → RR′NOH 2 + ) 定义。预计几乎所有简单的烷基和二烷基氮鎓离子单线态都会在没有可检测障碍的情况下重新排列,主要是通过 1,2 H 或烷基位移。甲基和N , N-二甲基氮鎓离子单线态可以表征为 PES 上的形式最小值。然而,这些物质对异构化表现出很小或不显着的障碍。包含烷基和共轭取代基(例如烷氧基、乙烯基或苯基)的二取代氮鎓离子对异构化表现出有意义的障碍,因此预计在溶液中具有非凡的寿命。相对于母体氮离子NH 2 +的情况,烷基基本上将单线态稳定到两种状态几乎简并的程度。其他与氮离子中心相互作用的基团按甲酰基 < 乙烯基 < 苯基 < 烷氧基 ~ 磺酰基 < 环丙基 ~ 环丁基的顺序降低 Δ E st 。后两个取代基通过形成非经典键与(单态)氮离子中心强烈相互作用,让人想起碳阳离子的平分环丙基碳酰离子情况。当在N-水合焓的背景下评估单线态稳定性时,发现排序为酰基<乙烯基<烷氧基<苯基<环丙基和环丁基。

































 京公网安备 11010802027423号
京公网安备 11010802027423号