Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intracrine Androgens Enhance Decidualization and Modulate Expression of Human Endometrial Receptivity Genes.
Scientific Reports ( IF 3.8 ) Pub Date : 2016-01-28 , DOI: 10.1038/srep19970 Douglas A Gibson 1 , Ioannis Simitsidellis 1 , Fiona L Cousins 1 , Hilary O D Critchley 2 , Philippa T K Saunders 1
Scientific Reports ( IF 3.8 ) Pub Date : 2016-01-28 , DOI: 10.1038/srep19970 Douglas A Gibson 1 , Ioannis Simitsidellis 1 , Fiona L Cousins 1 , Hilary O D Critchley 2 , Philippa T K Saunders 1
Affiliation
|
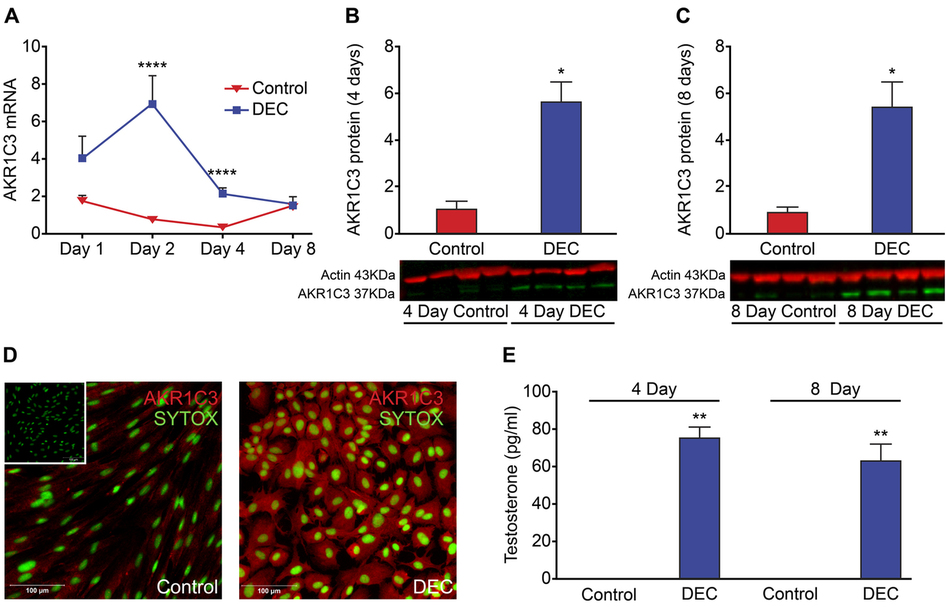
The endometrium is a complex, steroid-dependent tissue that undergoes dynamic cyclical remodelling. Transformation of stromal fibroblasts (ESC) into specialised secretory cells (decidualization) is fundamental to the establishment of a receptive endometrial microenvironment which can support and maintain pregnancy. Androgen receptors (AR) are present in ESC; in other tissues local metabolism of ovarian and adrenal-derived androgens regulate AR-dependent gene expression. We hypothesised that altered expression/activity of androgen biosynthetic enzymes would regulate tissue availability of bioactive androgens and the process of decidualization. Primary human ESC were treated in vitro for 1-8 days with progesterone and cAMP (decidualized) in the presence or absence of the AR antagonist flutamide. Time and treatment-dependent changes in genes essential for a) intra-tissue biosynthesis of androgens (5α-reductase/SRD5A1, aldo-keto reductase family 1 member C3/AKR1C3), b) establishment of endometrial decidualization (IGFBP1, prolactin) and c) endometrial receptivity (SPP1, MAOA, EDNRB) were measured. Decidualization of ESC resulted in significant time-dependent changes in expression of AKR1C3 and SRD5A1 and secretion of T/DHT. Addition of flutamide significantly reduced secretion of IGFBP1 and prolactin and altered the expression of endometrial receptivity markers. Intracrine biosynthesis of endometrial androgens during decidualization may play a key role in endometrial receptivity and offer a novel target for fertility treatment.
中文翻译:

内分泌雄激素增强蜕膜形成并调节人子宫内膜受体基因的表达。
子宫内膜是复杂的,类固醇依赖性组织,会经历动态的周期性重塑。基质成纤维细胞(ESC)转化为专门的分泌细胞(蜕膜化)对于建立可以支持和维持妊娠的子宫内膜微环境至关重要。雄激素受体(AR)存在于ESC中;在其他组织中,卵巢和肾上腺雄激素的局部代谢调节AR依赖性基因的表达。我们假设改变雄激素生物合成酶的表达/活性将调节生物活性雄激素的组织利用率和蜕膜化过程。在存在或不存在AR拮抗剂氟他胺的情况下,用孕酮和cAMP(蜕膜化)在体外对原代人ESC进行1-8天的治疗。时间和治疗依赖性的基因变化,这些基因对于a)组织内雄激素的生物合成(5α-还原酶/ SRD5A1,醛固酮还原酶家族1成员C3 / AKR1C3),b)子宫内膜蜕膜化(IGFBP1,催乳素)和c )测量子宫内膜的接受度(SPP1,MAOA,EDNRB)。ESC的蜕膜化导致AKR1C3和SRD5A1的表达以及T / DHT的分泌发生明显的时间依赖性变化。氟他胺的添加显着减少了IGFBP1和催乳激素的分泌,并改变了子宫内膜接受性标志物的表达。蜕膜化过程中子宫内膜雄激素的内分泌生物合成可能在子宫内膜的接受性中起关键作用,并为生育治疗提供了新的靶点。aldo-keto还原酶家族1成员C3 / AKR1C3),b)子宫内膜蜕膜化的建立(IGFBP1,催乳素)和c)子宫内膜的接受度(SPP1,MAOA,EDNRB)。ESC的蜕膜化导致AKR1C3和SRD5A1的表达以及T / DHT的分泌发生明显的时间依赖性变化。氟他胺的添加显着减少了IGFBP1和催乳激素的分泌,并改变了子宫内膜接受性标志物的表达。蜕膜化过程中子宫内膜雄激素的内分泌生物合成可能在子宫内膜的接受性中起关键作用,并为生育治疗提供了新的靶点。醛糖酮还原酶家族1成员C3 / AKR1C3),b)子宫内膜蜕膜化(IGFBP1,催乳激素)的建立和c)子宫内膜的接受度(SPP1,MAOA,EDNRB)。ESC的蜕膜化导致AKR1C3和SRD5A1的表达以及T / DHT的分泌发生明显的时间依赖性变化。氟他胺的添加显着减少了IGFBP1和催乳激素的分泌,并改变了子宫内膜接受性标志物的表达。蜕膜化过程中子宫内膜雄激素的内分泌生物合成可能在子宫内膜的接受性中起关键作用,并为生育治疗提供了新的靶点。ESC的蜕膜化导致AKR1C3和SRD5A1的表达以及T / DHT的分泌发生明显的时间依赖性变化。氟他胺的添加显着减少了IGFBP1和催乳激素的分泌,并改变了子宫内膜接受性标志物的表达。蜕膜化过程中子宫内膜雄激素的内分泌生物合成可能在子宫内膜的接受性中起关键作用,并为生育治疗提供了新的靶点。ESC的蜕膜化导致AKR1C3和SRD5A1的表达以及T / DHT的分泌发生明显的时间依赖性变化。氟他胺的添加显着减少了IGFBP1和催乳激素的分泌,并改变了子宫内膜接受性标志物的表达。蜕膜化过程中子宫内膜雄激素的内分泌生物合成可能在子宫内膜的接受性中起关键作用,并为生育治疗提供了新的靶点。
更新日期:2016-01-30
中文翻译:

内分泌雄激素增强蜕膜形成并调节人子宫内膜受体基因的表达。
子宫内膜是复杂的,类固醇依赖性组织,会经历动态的周期性重塑。基质成纤维细胞(ESC)转化为专门的分泌细胞(蜕膜化)对于建立可以支持和维持妊娠的子宫内膜微环境至关重要。雄激素受体(AR)存在于ESC中;在其他组织中,卵巢和肾上腺雄激素的局部代谢调节AR依赖性基因的表达。我们假设改变雄激素生物合成酶的表达/活性将调节生物活性雄激素的组织利用率和蜕膜化过程。在存在或不存在AR拮抗剂氟他胺的情况下,用孕酮和cAMP(蜕膜化)在体外对原代人ESC进行1-8天的治疗。时间和治疗依赖性的基因变化,这些基因对于a)组织内雄激素的生物合成(5α-还原酶/ SRD5A1,醛固酮还原酶家族1成员C3 / AKR1C3),b)子宫内膜蜕膜化(IGFBP1,催乳素)和c )测量子宫内膜的接受度(SPP1,MAOA,EDNRB)。ESC的蜕膜化导致AKR1C3和SRD5A1的表达以及T / DHT的分泌发生明显的时间依赖性变化。氟他胺的添加显着减少了IGFBP1和催乳激素的分泌,并改变了子宫内膜接受性标志物的表达。蜕膜化过程中子宫内膜雄激素的内分泌生物合成可能在子宫内膜的接受性中起关键作用,并为生育治疗提供了新的靶点。aldo-keto还原酶家族1成员C3 / AKR1C3),b)子宫内膜蜕膜化的建立(IGFBP1,催乳素)和c)子宫内膜的接受度(SPP1,MAOA,EDNRB)。ESC的蜕膜化导致AKR1C3和SRD5A1的表达以及T / DHT的分泌发生明显的时间依赖性变化。氟他胺的添加显着减少了IGFBP1和催乳激素的分泌,并改变了子宫内膜接受性标志物的表达。蜕膜化过程中子宫内膜雄激素的内分泌生物合成可能在子宫内膜的接受性中起关键作用,并为生育治疗提供了新的靶点。醛糖酮还原酶家族1成员C3 / AKR1C3),b)子宫内膜蜕膜化(IGFBP1,催乳激素)的建立和c)子宫内膜的接受度(SPP1,MAOA,EDNRB)。ESC的蜕膜化导致AKR1C3和SRD5A1的表达以及T / DHT的分泌发生明显的时间依赖性变化。氟他胺的添加显着减少了IGFBP1和催乳激素的分泌,并改变了子宫内膜接受性标志物的表达。蜕膜化过程中子宫内膜雄激素的内分泌生物合成可能在子宫内膜的接受性中起关键作用,并为生育治疗提供了新的靶点。ESC的蜕膜化导致AKR1C3和SRD5A1的表达以及T / DHT的分泌发生明显的时间依赖性变化。氟他胺的添加显着减少了IGFBP1和催乳激素的分泌,并改变了子宫内膜接受性标志物的表达。蜕膜化过程中子宫内膜雄激素的内分泌生物合成可能在子宫内膜的接受性中起关键作用,并为生育治疗提供了新的靶点。ESC的蜕膜化导致AKR1C3和SRD5A1的表达以及T / DHT的分泌发生明显的时间依赖性变化。氟他胺的添加显着减少了IGFBP1和催乳激素的分泌,并改变了子宫内膜接受性标志物的表达。蜕膜化过程中子宫内膜雄激素的内分泌生物合成可能在子宫内膜的接受性中起关键作用,并为生育治疗提供了新的靶点。






























 京公网安备 11010802027423号
京公网安备 11010802027423号