当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Forms of Mercury in Pyrite: Implications for Predicting Mercury Releases in Acid Mine Drainage Settings
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-08-31 , DOI: 10.1021/acs.est.8b02027 Alain Manceau 1 , Margarita Merkulova 2 , Magdalena Murdzek 2 , Valentina Batanova 1 , Rafal Baran 2 , Pieter Glatzel 2 , Binoy K. Saikia 3 , Dogan Paktunc 4 , Liliana Lefticariu 5, 6
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-08-31 , DOI: 10.1021/acs.est.8b02027 Alain Manceau 1 , Margarita Merkulova 2 , Magdalena Murdzek 2 , Valentina Batanova 1 , Rafal Baran 2 , Pieter Glatzel 2 , Binoy K. Saikia 3 , Dogan Paktunc 4 , Liliana Lefticariu 5, 6
Affiliation
|
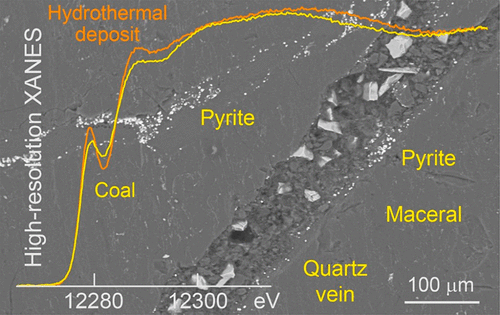
Pyrite (cubic FeS2) is the most abundant metal sulfide in nature and also the main host mineral of toxic mercury (Hg). Release of mercury in acid mine drainage resulting from the oxidative dissolution of pyrite in coal and ore and rock resulting from mining, processing, waste management, reclamation, and large construction activities is an ongoing environmental challenge. The fate of mercury depends on its chemical forms at the point source, which in turn depends on how it occurs in pyrite. Here, we show that pyrite in coal, sedimentary rocks, and hydrothermal ore deposits can host varying structural forms of Hg which can be identified with high energy-resolution XANES (HR-XANES) spectroscopy. Nominally divalent Hg is incorporated at the Fe site in pyrite from coal and at a marcasite-type Fe site in pyrite from sedimentary rocks. Distinction of the two Hg bonding environments offers a mean to detect microscopic marcasite inclusions (orthorhombic FeS2) in bulk pyrite. In epigenetic pyrite from Carlin-type Au deposit, up to 55 ± 6 at. % of the total Hg occurs as metacinnabar nanoparticles (β-HgSNP), with the remainder being substitutional at the Fe site. Pyritic mercury from Idrija-type Hg deposit (α-HgS ore) is partly divalent and substitutional and partly reduced into elemental form (liquid). Divalent mercury ions, mercury sulfide nanoparticles, and elemental mercury released by the oxidation of pyrite in acid mine drainage settings would have different environmental pathways. Our results could find important applications for designing control strategies of mercury released to land and water in mine-impacted watersheds.
中文翻译:

黄铁矿中汞的化学形式:在酸性矿山排水环境中预测汞释放的意义
硫铁矿(立方FeS 2)是自然界中最丰富的金属硫化物,也是有毒汞(Hg)的主要宿主矿物。开采,加工,废物管理,开垦和大型建筑活动导致的黄铁矿在煤,矿石和岩石中的氧化溶解导致酸性矿山排水中汞的释放是一项持续的环境挑战。汞的命运取决于点源的化学形式,而汞的化学形式又取决于其在黄铁矿中的生成方式。在这里,我们显示出煤,沉积岩和热液矿石矿床中的黄铁矿可以包含汞的各种结构形式,可以通过高能量分辨率XANES(HR-XANES)光谱鉴定。在煤的黄铁矿的Fe部位和沉积岩的黄铁矿的镁铁矿型Fe部位都掺入了名义上的二价汞。2)散装黄铁矿。在来自卡林型金矿床的后生黄铁矿中,高达55±6 at。总汞%发生是由于纳米颗粒metacinnabar(β-的HgS NP),剩余部分为在Fe位点取代。Idrija型Hg矿床(α-HgS矿石)中的硫铁矿汞部分为二价取代,部分还原为元素形式(液态)。在酸性矿山排水环境中,黄铁矿氧化释放的二价汞离子,硫化汞纳米粒子和元素汞将具有不同的环境途径。我们的结果可以在设计排雷影响的流域中向土地和水中释放的汞的控制策略中找到重要的应用。
更新日期:2018-09-01
中文翻译:

黄铁矿中汞的化学形式:在酸性矿山排水环境中预测汞释放的意义
硫铁矿(立方FeS 2)是自然界中最丰富的金属硫化物,也是有毒汞(Hg)的主要宿主矿物。开采,加工,废物管理,开垦和大型建筑活动导致的黄铁矿在煤,矿石和岩石中的氧化溶解导致酸性矿山排水中汞的释放是一项持续的环境挑战。汞的命运取决于点源的化学形式,而汞的化学形式又取决于其在黄铁矿中的生成方式。在这里,我们显示出煤,沉积岩和热液矿石矿床中的黄铁矿可以包含汞的各种结构形式,可以通过高能量分辨率XANES(HR-XANES)光谱鉴定。在煤的黄铁矿的Fe部位和沉积岩的黄铁矿的镁铁矿型Fe部位都掺入了名义上的二价汞。2)散装黄铁矿。在来自卡林型金矿床的后生黄铁矿中,高达55±6 at。总汞%发生是由于纳米颗粒metacinnabar(β-的HgS NP),剩余部分为在Fe位点取代。Idrija型Hg矿床(α-HgS矿石)中的硫铁矿汞部分为二价取代,部分还原为元素形式(液态)。在酸性矿山排水环境中,黄铁矿氧化释放的二价汞离子,硫化汞纳米粒子和元素汞将具有不同的环境途径。我们的结果可以在设计排雷影响的流域中向土地和水中释放的汞的控制策略中找到重要的应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号