当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
F-actin-rich contractile endothelial pores prevent vascular leakage during leukocyte diapedesis through local RhoA signalling.
Nature Communications ( IF 14.7 ) Pub Date : 2016-Jan-27 , DOI: 10.1038/ncomms10493 Niels Heemskerk , Lilian Schimmel , Chantal Oort , Jos van Rijssel , Taofei Yin , Bin Ma , Jakobus van Unen , Bettina Pitter , Stephan Huveneers , Joachim Goedhart , Yi Wu , Eloi Montanez , Abigail Woodfin , Jaap D. van Buul
Nature Communications ( IF 14.7 ) Pub Date : 2016-Jan-27 , DOI: 10.1038/ncomms10493 Niels Heemskerk , Lilian Schimmel , Chantal Oort , Jos van Rijssel , Taofei Yin , Bin Ma , Jakobus van Unen , Bettina Pitter , Stephan Huveneers , Joachim Goedhart , Yi Wu , Eloi Montanez , Abigail Woodfin , Jaap D. van Buul
|
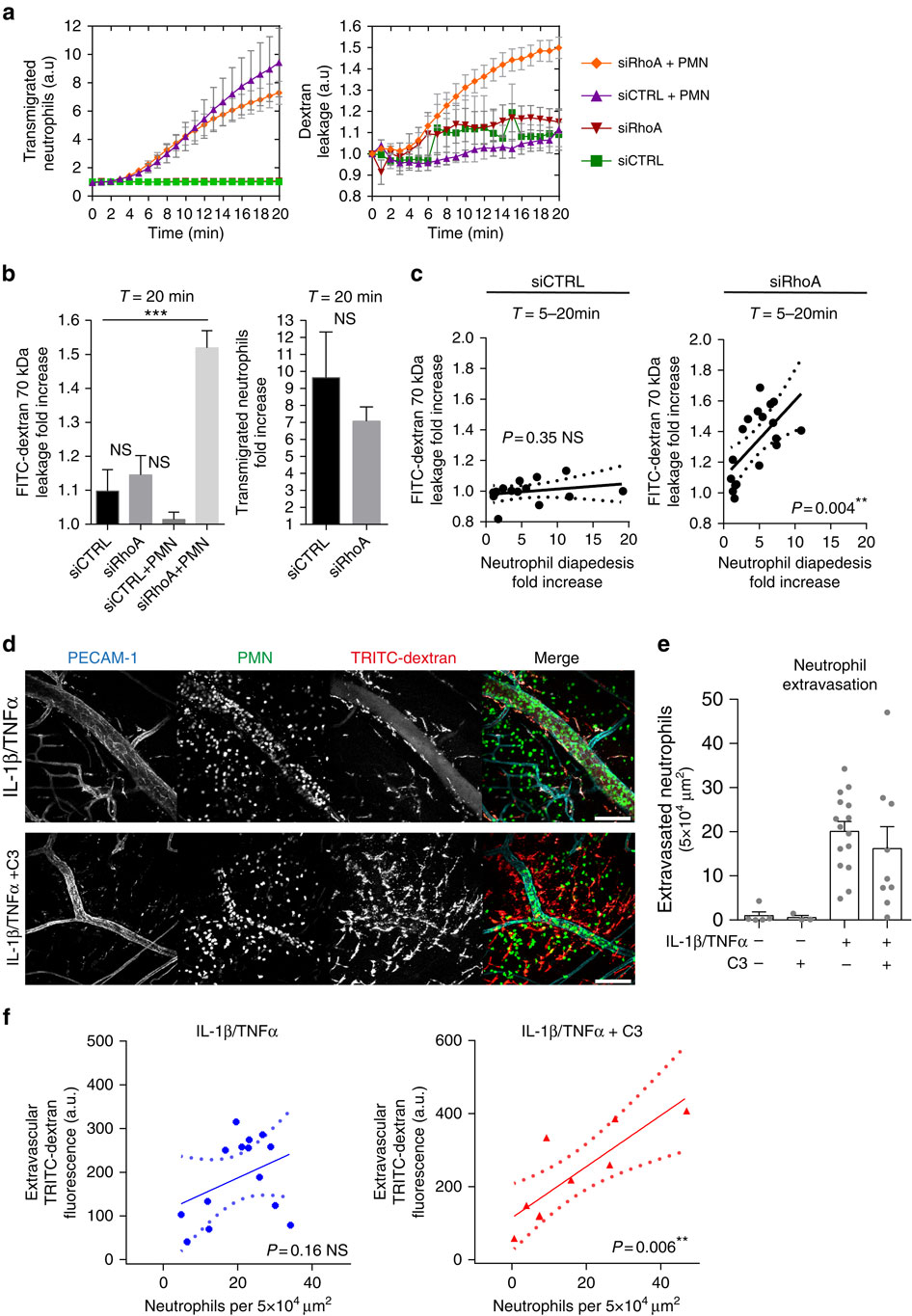
During immune surveillance and inflammation, leukocytes exit the vasculature through transient openings in the endothelium without causing plasma leakage. However, the exact mechanisms behind this intriguing phenomenon are still unknown. Here we report that maintenance of endothelial barrier integrity during leukocyte diapedesis requires local endothelial RhoA cycling. Endothelial RhoA depletion in vitro or Rho inhibition in vivo provokes neutrophil-induced vascular leakage that manifests during the physical movement of neutrophils through the endothelial layer. Local RhoA activation initiates the formation of contractile F-actin structures that surround emigrating neutrophils. These structures that surround neutrophil-induced endothelial pores prevent plasma leakage through actomyosin-based pore confinement. Mechanistically, we found that the initiation of RhoA activity involves ICAM-1 and the Rho GEFs Ect2 and LARG. In addition, regulation of actomyosin-based endothelial pore confinement involves ROCK2b, but not ROCK1. Thus, endothelial cells assemble RhoA-controlled contractile F-actin structures around endothelial pores that prevent vascular leakage during leukocyte extravasation.
中文翻译:

富含F-肌动蛋白的收缩性内皮细胞孔可通过局部RhoA信号传导阻止白细胞尿布分离期间的血管渗漏。
在免疫监视和炎症过程中,白细胞通过内皮中的瞬时开口离开脉管系统,而不会引起血浆渗漏。但是,这种有趣现象背后的确切机制仍是未知的。在这里,我们报告说,在白细胞浸透过程中维持内皮屏障的完整性需要局部内皮RhoA循环。体外内皮RhoA耗竭或体内Rho抑制会引起中性粒细胞诱导的血管渗漏,这在中性粒细胞通过内皮层的物理运动过程中表现出来。局部RhoA激活引发围绕迁移的中性粒细胞的收缩性F-肌动蛋白结构的形成。这些围绕中性粒细胞诱导的内皮孔的结构通过基于肌动球蛋白的孔限制来防止血浆泄漏。机械上,我们发现RhoA活性的启动涉及ICAM-1和Rho GEFs Ect2和LARG。此外,基于放线菌素的内皮细胞孔的调节涉及ROCK2b,但不涉及ROCK1。因此,内皮细胞在内皮孔周围组装RhoA控制的收缩性F-肌动蛋白结构,从而防止白细胞外渗期间的血管渗漏。
更新日期:2016-01-30
中文翻译:

富含F-肌动蛋白的收缩性内皮细胞孔可通过局部RhoA信号传导阻止白细胞尿布分离期间的血管渗漏。
在免疫监视和炎症过程中,白细胞通过内皮中的瞬时开口离开脉管系统,而不会引起血浆渗漏。但是,这种有趣现象背后的确切机制仍是未知的。在这里,我们报告说,在白细胞浸透过程中维持内皮屏障的完整性需要局部内皮RhoA循环。体外内皮RhoA耗竭或体内Rho抑制会引起中性粒细胞诱导的血管渗漏,这在中性粒细胞通过内皮层的物理运动过程中表现出来。局部RhoA激活引发围绕迁移的中性粒细胞的收缩性F-肌动蛋白结构的形成。这些围绕中性粒细胞诱导的内皮孔的结构通过基于肌动球蛋白的孔限制来防止血浆泄漏。机械上,我们发现RhoA活性的启动涉及ICAM-1和Rho GEFs Ect2和LARG。此外,基于放线菌素的内皮细胞孔的调节涉及ROCK2b,但不涉及ROCK1。因此,内皮细胞在内皮孔周围组装RhoA控制的收缩性F-肌动蛋白结构,从而防止白细胞外渗期间的血管渗漏。































 京公网安备 11010802027423号
京公网安备 11010802027423号