Redox Biology ( IF 10.7 ) Pub Date : 2018-08-30 , DOI: 10.1016/j.redox.2018.08.020 Luisa B Maia 1 , José J G Moura 1
|
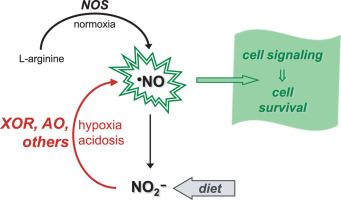
Nitric oxide radical (NO) is a signaling molecule involved in several physiological and pathological processes and a new nitrate-nitrite-NO pathway has emerged as a physiological alternative to the "classic" pathway of NO formation from L-arginine. Since the late 1990s, it has become clear that nitrite can be reduced back to NO under hypoxic/anoxic conditions and exert a significant cytoprotective action in vivo under challenging conditions. To reduce nitrite to NO, mammalian cells can use different metalloproteins that are present in cells to perform other functions, including several heme proteins and molybdoenzymes, comprising what we denominated as the "non-dedicated nitrite reductases". Herein, we will review the current knowledge on two of those "non-dedicated nitrite reductases", the molybdoenzymes xanthine oxidoreductase and aldehyde oxidase, discussing the in vitro and in vivo studies to provide the current picture of the role of these enzymes on the NO metabolism in humans.
中文翻译:

将黄嘌呤氧化还原酶和醛氧化酶置于NO代谢图上:钼酶还原亚硝酸盐
一氧化氮自由基 (NO) 是参与多种生理和病理过程的信号分子,一种新的硝酸盐-亚硝酸盐-NO 途径已成为L-精氨酸形成 NO 的“经典”途径的生理替代途径。自 20 世纪 90 年代末以来,人们已经清楚亚硝酸盐可以在缺氧/缺氧条件下还原为 NO,并在挑战性条件下在体内发挥显着的细胞保护作用。为了将亚硝酸盐还原为一氧化氮,哺乳动物细胞可以使用细胞中存在的不同金属蛋白来执行其他功能,包括几种血红素蛋白和钼酶,其中包括我们称为“非专用亚硝酸盐还原酶”的物质。在此,我们将回顾目前对其中两种“非专用亚硝酸盐还原酶”(钼酶黄嘌呤氧化还原酶和醛氧化酶)的了解,讨论体外和体内研究,以提供这些酶对 NO 作用的当前情况。人体的新陈代谢。































 京公网安备 11010802027423号
京公网安备 11010802027423号