当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Establishing new scaling relations on two-dimensional MXenes for CO2 electroreduction†
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-08-30 00:00:00 , DOI: 10.1039/c8ta06567e Albertus D. Handoko 1, 2, 3, 4 , Khoong Hong Khoo 2, 4, 5, 6 , Teck Leong Tan 2, 4, 5, 6 , Hongmei Jin 2, 4, 5, 6 , Zhi Wei Seh 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-08-30 00:00:00 , DOI: 10.1039/c8ta06567e Albertus D. Handoko 1, 2, 3, 4 , Khoong Hong Khoo 2, 4, 5, 6 , Teck Leong Tan 2, 4, 5, 6 , Hongmei Jin 2, 4, 5, 6 , Zhi Wei Seh 1, 2, 3, 4
Affiliation
|
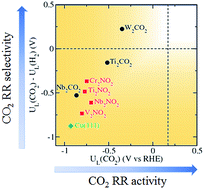
Electrochemical reduction of CO2 enables the utilisation and conversion of CO2 greenhouse gas into valuable fuels and chemicals. Transition metals, copper in particular, are most widely investigated as catalysts due to their ability to convert CO2 into a multitude of value-added carbon products. However, linear scaling relations prevent similarly-bound reaction intermediates (*CO, *CHO) from being stabilised independently on the surface, therefore severely limiting the activity and overpotential of transition metal catalysts. Here, we present a theoretical study of two-dimensional transition metal carbides and nitrides (MXenes) as promising electrocatalysts for the reduction of CO2 to CH4. A different CO2 reduction pathway through –H coordinated *HCOOH was discovered on O-terminated MXene catalysts due to generally weaker *CO binding. New scaling relations were established based on the alternating –C and –H coordination of the intermediates along the minimum energy pathway. Importantly, we found that the limiting potential of the MXene catalysts is determined by the binding energies of *COOH and/or *HCOOH which can be tuned independently, allowing significantly lower overpotential to be achieved compared to transition metals. In particular, two promising MXenes with theoretical overpotentials of 0.52 and 0.69 V, and competitive selectivity with respect to hydrogen evolution, were identified.
中文翻译:

在二维MXene上建立用于CO 2电解还原的新比例关系†
CO 2的电化学还原使得能够利用CO 2温室气体并将其转化为有价值的燃料和化学物质。过渡金属,尤其是铜,由于其能够将CO 2转化为多种增值的碳产品而被广泛地用作催化剂。然而,线性比例关系阻止相似结合的反应中间体(* CO,* CHO)独立地稳定在表面上,因此严重限制了过渡金属催化剂的活性和过电势。在这里,我们介绍了二维过渡金属碳化物和氮化物(MXenes)作为将CO 2还原为CH 4的有希望的电催化剂的理论研究。不同的CO在O封端的MXene催化剂上发现了通过–H配位的* HCOOH的2还原途径,因为* CO的结合通常较弱。基于最小能量路径上中间体的–C和–H交替配位,建立了新的比例关系。重要的是,我们发现MXene催化剂的极限电势由* COOH和/或* HCOOH的键能决定,该键能可以独立调节,与过渡金属相比,可以实现更低的超电势。尤其是,鉴定出了两个有前途的MXene,它们的理论过电势分别为0.52和0.69 V,并且相对于氢释放具有竞争选择性。
更新日期:2018-08-30
中文翻译:

在二维MXene上建立用于CO 2电解还原的新比例关系†
CO 2的电化学还原使得能够利用CO 2温室气体并将其转化为有价值的燃料和化学物质。过渡金属,尤其是铜,由于其能够将CO 2转化为多种增值的碳产品而被广泛地用作催化剂。然而,线性比例关系阻止相似结合的反应中间体(* CO,* CHO)独立地稳定在表面上,因此严重限制了过渡金属催化剂的活性和过电势。在这里,我们介绍了二维过渡金属碳化物和氮化物(MXenes)作为将CO 2还原为CH 4的有希望的电催化剂的理论研究。不同的CO在O封端的MXene催化剂上发现了通过–H配位的* HCOOH的2还原途径,因为* CO的结合通常较弱。基于最小能量路径上中间体的–C和–H交替配位,建立了新的比例关系。重要的是,我们发现MXene催化剂的极限电势由* COOH和/或* HCOOH的键能决定,该键能可以独立调节,与过渡金属相比,可以实现更低的超电势。尤其是,鉴定出了两个有前途的MXene,它们的理论过电势分别为0.52和0.69 V,并且相对于氢释放具有竞争选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号