当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Insights into Corrosion of Ruthenium and Ruthenium Oxide Nanoparticles in Acidic Media
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-04-24 00:00:00 , DOI: 10.1021/acs.jpcc.5b01832 Nejc Hodnik 1 , Primož Jovanovič 1 , Andraž Pavlišič 1 , Barbara Jozinović 1 , Milena Zorko 1 , Marjan Bele 1 , Vid Simon Šelih 1 , Martin Šala 1 , Samo Hočevar 1 , Miran Gaberšček 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-04-24 00:00:00 , DOI: 10.1021/acs.jpcc.5b01832 Nejc Hodnik 1 , Primož Jovanovič 1 , Andraž Pavlišič 1 , Barbara Jozinović 1 , Milena Zorko 1 , Marjan Bele 1 , Vid Simon Šelih 1 , Martin Šala 1 , Samo Hočevar 1 , Miran Gaberšček 1
Affiliation
|
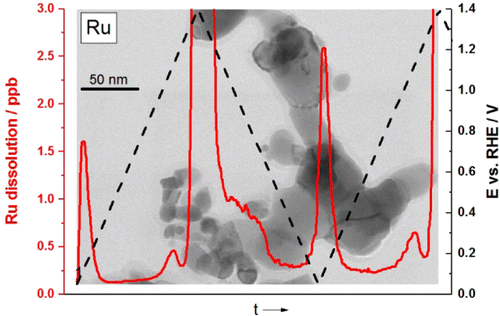
The dissolution behaviors of Ru and ruthenium oxide nanoparticles in acidic media were studied for the first time using highly sensitive in situ measurements of concentration by inductively coupled plasma mass spectrometry (ICP-MS). Online time- and potential-resolved electrochemical dissolution profiles revealed novel corrosion features (signals) in the potential window from 0 to ∼1.4 V, where known severe dissolution due to the oxygen evolution reaction (OER) takes place. Most of the features follow the thermodynamic changes of the Ru oxidation/reduction state, which consequently trigger so-called transient dissolution. An as-synthesized Ru sample was found to exhibit an order-of-magnitude higher dissolution rate than an electrochemically oxidized amorphous Ru sample. The latter, in turn, dissolved about 10 times faster than rutile RuO2. The observed OER activity was in an inverse relationship with the measured dissolution. Disagreement was found with the general assumption that the onset of the OER should coincide with the onset of Ru dissolution. Interestingly, in all samples, Ru dissolution was observed at about 0.17 V lower potentials than the OER. The present results are relevant for various energy-conversion and -storage devices such as proton-exchange membrane electrolyzers, low-temperature fuel cells, reverse fuel cells, supercapacitors, batteries, and photocatalysts that can contain Ru as an active component.
中文翻译:

钌和氧化钌纳米粒子在酸性介质中腐蚀的新见解
使用电感耦合等离子体质谱法(ICP-MS)进行浓度的高度灵敏的原位测量,首次研究了钌和氧化钌纳米颗粒在酸性介质中的溶解行为。在线时间和电位分辨的电化学溶解曲线揭示了在电位窗口中从0到〜1.4 V的新腐蚀特征(信号),其中发生了已知的由于氧释放反应(OER)引起的严重溶解。大多数特征遵循Ru氧化/还原态的热力学变化,从而触发所谓的瞬时溶解。发现合成后的Ru样品比电化学氧化的无定形Ru样品具有更高的数量级溶出速率。反过来,后者的溶解速度比金红石型RuO快约10倍2。观察到的OER活性与测得的溶出度成反比关系。在普遍假设中,OER的发生应与Ru的溶解发生相一致,这是存在分歧的。有趣的是,在所有样品中,在比OER低约0.17 V的电位下观察到Ru溶解。本结果与各种能量转换和存储装置有关,例如质子交换膜电解槽,低温燃料电池,反向燃料电池,超级电容器,电池和可以包含Ru作为活性成分的光催化剂。
更新日期:2015-04-24
中文翻译:

钌和氧化钌纳米粒子在酸性介质中腐蚀的新见解
使用电感耦合等离子体质谱法(ICP-MS)进行浓度的高度灵敏的原位测量,首次研究了钌和氧化钌纳米颗粒在酸性介质中的溶解行为。在线时间和电位分辨的电化学溶解曲线揭示了在电位窗口中从0到〜1.4 V的新腐蚀特征(信号),其中发生了已知的由于氧释放反应(OER)引起的严重溶解。大多数特征遵循Ru氧化/还原态的热力学变化,从而触发所谓的瞬时溶解。发现合成后的Ru样品比电化学氧化的无定形Ru样品具有更高的数量级溶出速率。反过来,后者的溶解速度比金红石型RuO快约10倍2。观察到的OER活性与测得的溶出度成反比关系。在普遍假设中,OER的发生应与Ru的溶解发生相一致,这是存在分歧的。有趣的是,在所有样品中,在比OER低约0.17 V的电位下观察到Ru溶解。本结果与各种能量转换和存储装置有关,例如质子交换膜电解槽,低温燃料电池,反向燃料电池,超级电容器,电池和可以包含Ru作为活性成分的光催化剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号