当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective and Stereoselective Reductive Aziridinium Ring Cleavage Leading to Azabicyclodecane Architecture: Enantioselective Synthesis of (+)-Subincanadine F
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-30 00:00:00 , DOI: 10.1021/acs.joc.8b02113 Manojkumar G. Kalshetti 1, 2 , Narshinha P. Argade 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-30 00:00:00 , DOI: 10.1021/acs.joc.8b02113 Manojkumar G. Kalshetti 1, 2 , Narshinha P. Argade 1, 2
Affiliation
|
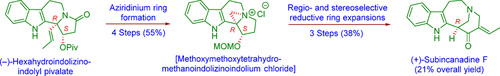
Enantioselective synthesis of cytotoxic indole alkaloid (+)-subincanadine F was accomplished starting from the corresponding (S)-acetoxysuccinimide via aziridinium ring formation and its reductive ring expansion route. Regioselective and stereoselective reductive aziridinium carbon–nitrogen bond cleavage comprising ring expansions was a key step. The (S)-OMOM protection of the hydroxyl moiety adjacent to a benzylic carbon of an in situ formed aziridinium system was necessary for lithium borohydride-induced reductive ring expansions, and it also served as a latent source of an essential ketone carbonyl group for the generation of an α,β-conjugated system.
中文翻译:

区域选择性和立体选择性还原氮杂环丁烷环断裂导致氮杂双环癸烷体系结构:(+)-Subincanadine F的对映选择性合成
对映体选择性合成细胞毒性吲哚生物碱(+)-incincanadine F是从相应的(S)-乙酰氧基琥珀酰亚胺通过叠氮鎓环的形成及其还原性环的扩展途径而完成的。区域选择性和立体选择性还原的叠氮基氮-碳氮键裂解(包括环的扩展)是关键的一步。原位形成的叠氮鎓体系的苄基碳附近的羟基部分的(S)-OMOM保护对于硼氢化锂诱导的还原环扩张是必需的,并且它还充当了潜在的酮基羰基羰基的潜在来源。 α,β共轭体系的产生。
更新日期:2018-08-30
中文翻译:

区域选择性和立体选择性还原氮杂环丁烷环断裂导致氮杂双环癸烷体系结构:(+)-Subincanadine F的对映选择性合成
对映体选择性合成细胞毒性吲哚生物碱(+)-incincanadine F是从相应的(S)-乙酰氧基琥珀酰亚胺通过叠氮鎓环的形成及其还原性环的扩展途径而完成的。区域选择性和立体选择性还原的叠氮基氮-碳氮键裂解(包括环的扩展)是关键的一步。原位形成的叠氮鎓体系的苄基碳附近的羟基部分的(S)-OMOM保护对于硼氢化锂诱导的还原环扩张是必需的,并且它还充当了潜在的酮基羰基羰基的潜在来源。 α,β共轭体系的产生。






























 京公网安备 11010802027423号
京公网安备 11010802027423号