当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
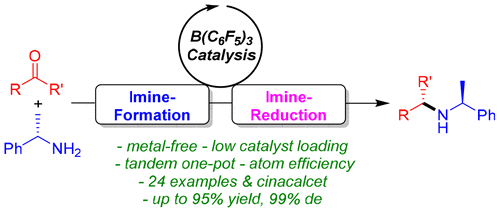
B(C6F5)3-Catalyzed Asymmetric Reductive Amination of Ketones with Ammonia Borane
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acs.joc.8b01362
Zhentao Pan 1 , Leixin Shen 1 , Dingguo Song 1 , Zhen Xie 1 , Fei Ling 1 , Weihui Zhong 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acs.joc.8b01362
Zhentao Pan 1 , Leixin Shen 1 , Dingguo Song 1 , Zhen Xie 1 , Fei Ling 1 , Weihui Zhong 1
Affiliation
|
The first example of metal-free B(C6F5)3-catalyzed asymmetric reduction amination of ketones with chiral α-methylbenzylamine (α-MBA) using ammonia borane as the reductant is reported. This one-pot method has a broad substrate scope and provides various chiral amines in 81–95% yield with 80–99% de. This protocol was further applied in the total synthesis of cinacalcet.
中文翻译:

B(C 6 F 5)3-氨硼烷催化的酮不对称还原胺化
报道了使用氨硼烷作为还原剂,用手性α-甲基苄胺(α-MBA)进行的无金属的B(C 6 F 5)3催化的酮的不对称还原胺化的第一个实例。这种一锅法具有广泛的底物范围,并以81-95%的收率和80-99%的de提供了各种手性胺。该方案进一步应用于西那卡塞的全合成中。
更新日期:2018-08-29
中文翻译:

B(C 6 F 5)3-氨硼烷催化的酮不对称还原胺化
报道了使用氨硼烷作为还原剂,用手性α-甲基苄胺(α-MBA)进行的无金属的B(C 6 F 5)3催化的酮的不对称还原胺化的第一个实例。这种一锅法具有广泛的底物范围,并以81-95%的收率和80-99%的de提供了各种手性胺。该方案进一步应用于西那卡塞的全合成中。































 京公网安备 11010802027423号
京公网安备 11010802027423号