当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mixed Dimetallic Cluster Fullerenes: [email protected]C3v(8)-C82 and ScGdC2@C2v(9)-C82
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acs.inorgchem.8b01646 Wei Yang 1 , Laura Abella 2 , Yaofeng Wang 1 , Xiaohong Li 1 , Jiali Gu 1 , Josep M. Poblet 2 , Antonio Rodríguez-Fortea 2 , Ning Chen 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acs.inorgchem.8b01646 Wei Yang 1 , Laura Abella 2 , Yaofeng Wang 1 , Xiaohong Li 1 , Jiali Gu 1 , Josep M. Poblet 2 , Antonio Rodríguez-Fortea 2 , Ning Chen 1
Affiliation
|
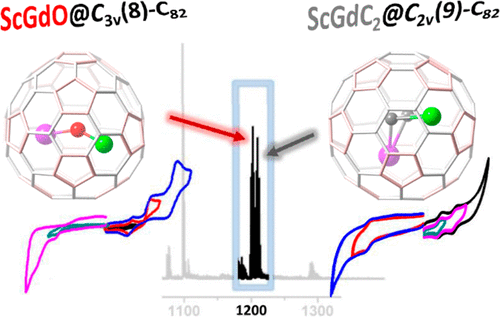
Mixed-metal cluster fullerenes have been extensively studied in recent years for their rich structural variability of the encaged clusters and have shown great potential in applied studies such as biomedicine and molecular electronic devices. However, the studies in this field have mostly concentrated on the nitride cluster fullerene, and very few other types of mixed-metal cluster fullerenes have been reported so far. Herein, we report the synthesis and isolation of the first mixed-metal oxide cluster fullerene, [email protected]82, and a novel mixed dimetallic carbide cluster fullerene, ScGdC2@C82. Spectroscopic and electrochemical studies combined with density functional theory (DFT) calculations assigned the molecular structure of the two cluster fullerenes (CFs) to [email protected]C3v(8)-C82 and ScGdC2@C2v(9)-C82, respectively. DFT calculations also suggested that these two mixed-metal clusters are likely to be found in any of the three isolated pentagon rule Cs(6)-C82, C3v(8)-C82 and/or C2v(9)-C82 cages. The electrochemical studies show that the electrochemical gap of [email protected]C3v(8)-C82 and ScGdC2@C2v(9)-C82 are 1.49 and 1.08 V. Moreover, comparative studies of [email protected]C3v(8)-C82 and Sc2[email protected]C3v(8)-C82, ScGdC2@C2v(9)-C82 and Sc2C2@C2v(9)-C82 showed that, despite their close structural resemblance, the replacement of one Sc ion by a Gd ion resulted in notable changes in their electrochemical behaviors as well as their 45Sc NMR spectra.
中文翻译:

混合双金属簇富勒烯:[受电子邮件保护] C 3v(8)-C 82和ScGdC 2 @ C 2v(9)-C 82
近年来,混合金属团簇富勒烯因其包裹团簇的丰富结构变异性而被广泛研究,并在诸如生物医学和分子电子装置的应用研究中显示出巨大潜力。但是,该领域的研究主要集中在氮化物簇状富勒烯上,到目前为止,几乎没有其他类型的混合金属簇状富勒烯被报道。在这里,我们报告合成和分离的第一个混合金属氧化物簇富勒烯[电子邮件保护] 82和一个新型的混合双金属碳化物簇富勒烯ScGdC 2 @C 82。光谱和电化学研究与密度泛函理论(DFT)计算相结合,将两个簇富勒烯(CF)的分子结构分配给[电子邮件保护] C 3v(8)-C 82和ScGdC 2 @ C 2v(9)-C 82, 分别。DFT计算还表明,这两个混合金属簇很可能在三个孤立的五边形规则C s(6)-C 82,C 3v(8)-C 82和/或C 2v(9)-中找到C 82笼子。电化学研究表明[受电子邮件保护的] C 3v(8)-C 82和ScGdC 2 @ C 2v(9)-C 82的电化学间隙为1.49和1.08V 。此外,[受电子邮件保护的] C 3v的比较研究(8)-C 82和Sc 2 [受电子邮件保护] C 3v(8)-C 82,ScGdC 2 @ C 2v(9)-C 82和Sc 2 C 2 @ C 2v(9)-C 82结果表明,尽管它们具有相似的结构相似性,但用Gd离子代替一个Sc离子后,其电化学行为以及45 Sc NMR谱图发生了显着变化。
更新日期:2018-08-29
中文翻译:

混合双金属簇富勒烯:[受电子邮件保护] C 3v(8)-C 82和ScGdC 2 @ C 2v(9)-C 82
近年来,混合金属团簇富勒烯因其包裹团簇的丰富结构变异性而被广泛研究,并在诸如生物医学和分子电子装置的应用研究中显示出巨大潜力。但是,该领域的研究主要集中在氮化物簇状富勒烯上,到目前为止,几乎没有其他类型的混合金属簇状富勒烯被报道。在这里,我们报告合成和分离的第一个混合金属氧化物簇富勒烯[电子邮件保护] 82和一个新型的混合双金属碳化物簇富勒烯ScGdC 2 @C 82。光谱和电化学研究与密度泛函理论(DFT)计算相结合,将两个簇富勒烯(CF)的分子结构分配给[电子邮件保护] C 3v(8)-C 82和ScGdC 2 @ C 2v(9)-C 82, 分别。DFT计算还表明,这两个混合金属簇很可能在三个孤立的五边形规则C s(6)-C 82,C 3v(8)-C 82和/或C 2v(9)-中找到C 82笼子。电化学研究表明[受电子邮件保护的] C 3v(8)-C 82和ScGdC 2 @ C 2v(9)-C 82的电化学间隙为1.49和1.08V 。此外,[受电子邮件保护的] C 3v的比较研究(8)-C 82和Sc 2 [受电子邮件保护] C 3v(8)-C 82,ScGdC 2 @ C 2v(9)-C 82和Sc 2 C 2 @ C 2v(9)-C 82结果表明,尽管它们具有相似的结构相似性,但用Gd离子代替一个Sc离子后,其电化学行为以及45 Sc NMR谱图发生了显着变化。















































 京公网安备 11010802027423号
京公网安备 11010802027423号