当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox Potential‐Dependent Formation of an Unusual His–Trp Bond in Bilirubin Oxidase
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-11-15 , DOI: 10.1002/chem.201803798 Mahfuza Akter 1 , Takaki Tokiwa 2 , Mitsuo Shoji 3 , Koji Nishikawa 1 , Yasuteru Shigeta 3 , Takeshi Sakurai 4 , Yoshiki Higuchi 1, 5 , Kunishige Kataoka 4 , Naoki Shibata 1, 5
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-11-15 , DOI: 10.1002/chem.201803798 Mahfuza Akter 1 , Takaki Tokiwa 2 , Mitsuo Shoji 3 , Koji Nishikawa 1 , Yasuteru Shigeta 3 , Takeshi Sakurai 4 , Yoshiki Higuchi 1, 5 , Kunishige Kataoka 4 , Naoki Shibata 1, 5
Affiliation
|
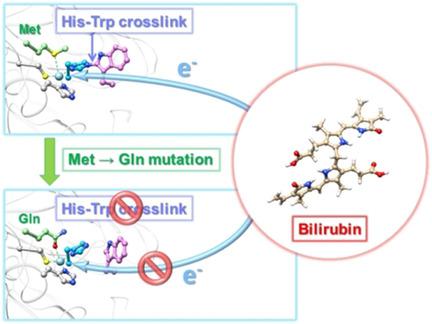
Bilirubin oxidase (BOD) belongs to the family of blue multicopper oxidases, and catalyzes the concomitant oxidation of bilirubin to biliverdin and the reduction of molecular oxygen to water via a four‐electron reduction system. The active sites of BOD comprise four copper atoms; type I copper (T1Cu) forms a mononuclear site, and a cluster of three copper atoms forms a trinuclear center. In the present study, we determined the high‐resolution crystal structures of BOD from the fungus Myrothecium verrucaria. We investigated wild‐type (WT) BOD and a BOD mutant called Met467Gln, which is inactive against bilirubin. The structures revealed that a novel post‐translational crosslink between Trp396 and His398 is formed in the vicinity of the T1Cu site in WT BOD, whereas it is absent in the Met467Gln mutant. Our structural and computational studies suggest that the His–Trp crosslink adjusts the redox potential of T1Cu to that of bilirubin to efficiently abstract electrons from the substrate.
中文翻译:

胆红素氧化酶中异常的His-Trp键的氧化还原电位依赖性形成
胆红素氧化酶(BOD)属于蓝色多铜氧化酶家族,可催化胆红素氧化为胆绿素,并通过四电子还原系统将分子氧还原为水。BOD的活性位点包含四个铜原子。I型铜(T1Cu)形成一个单核位,三个铜原子簇形成一个三核中心。在当前的研究中,我们确定了真菌Myrothecium verrucaria的BOD的高分辨率晶体结构。。我们研究了野生型(WT)BOD和一个称为Met467Gln的BOD突变体,该突变体对胆红素没有活性。结构表明,在WT BOD的T1Cu位点附近形成了Trp396和His398之间的新型翻译后交联,而在Met467Gln突变体中则不存在。我们的结构和计算研究表明,His-Trp交联键可将T1Cu的氧化还原电位调节为胆红素的氧化还原电位,以有效地从底物中提取电子。
更新日期:2018-11-15
中文翻译:

胆红素氧化酶中异常的His-Trp键的氧化还原电位依赖性形成
胆红素氧化酶(BOD)属于蓝色多铜氧化酶家族,可催化胆红素氧化为胆绿素,并通过四电子还原系统将分子氧还原为水。BOD的活性位点包含四个铜原子。I型铜(T1Cu)形成一个单核位,三个铜原子簇形成一个三核中心。在当前的研究中,我们确定了真菌Myrothecium verrucaria的BOD的高分辨率晶体结构。。我们研究了野生型(WT)BOD和一个称为Met467Gln的BOD突变体,该突变体对胆红素没有活性。结构表明,在WT BOD的T1Cu位点附近形成了Trp396和His398之间的新型翻译后交联,而在Met467Gln突变体中则不存在。我们的结构和计算研究表明,His-Trp交联键可将T1Cu的氧化还原电位调节为胆红素的氧化还原电位,以有效地从底物中提取电子。































 京公网安备 11010802027423号
京公网安备 11010802027423号