当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh(III)-Catalyzed Oxidative Annulation of Isoquinolones with Diazoketoesters Featuring an in Situ Deacylation: Synthesis of Isoindoloisoquinolones and Their Transformation to Rosettacin Analogues
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acs.joc.8b01982 Shenghai Guo 1 , Lincong Sun 1 , Fang Wang 1 , Xinying Zhang 1 , Xuesen Fan 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acs.joc.8b01982 Shenghai Guo 1 , Lincong Sun 1 , Fang Wang 1 , Xinying Zhang 1 , Xuesen Fan 1
Affiliation
|
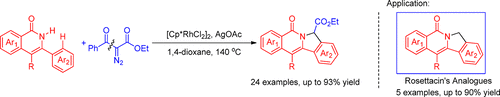
A novel and practical procedure for the preparation of isoindolo[2,1-b]isoquinoline-7-carboxylate derivatives through a Rh(III)-catalyzed oxidative [4 + 1] cycloaddition of isoquinolones with diazoketoesters followed by an in situ deacylation reaction is disclosed. Intriguingly, the title compounds could be easily converted into isoindolo[2,1-b]isoquinolin-5(7H)-ones via de-esterification, which are rosettacin analogues and frequently found in various natural alkaloids and synthetic drug molecules.
中文翻译:

Rh(III)催化的异喹啉酮与具有原位脱酰基反应的二氮杂酮酸酯的氧化环化反应:异吲哚异喹啉酮的合成及其向Rosettacin类似物的转化
通过Rh(III)催化的异喹诺酮与重氮酮酸酯的氧化[4 +1]环加成,然后进行原位脱酰反应,制备异吲哚并[2,1 - b ]异喹啉-7-羧酸酯衍生物的新颖实用的方法是披露。有趣的是,标题化合物可以很容易地通过去酯化反应转变为异吲哚并[2,1 - b ]异喹啉-5(7 H)-,这是一种罗塞他汀类似物,经常在各种天然生物碱和合成药物分子中发现。
更新日期:2018-08-29
中文翻译:

Rh(III)催化的异喹啉酮与具有原位脱酰基反应的二氮杂酮酸酯的氧化环化反应:异吲哚异喹啉酮的合成及其向Rosettacin类似物的转化
通过Rh(III)催化的异喹诺酮与重氮酮酸酯的氧化[4 +1]环加成,然后进行原位脱酰反应,制备异吲哚并[2,1 - b ]异喹啉-7-羧酸酯衍生物的新颖实用的方法是披露。有趣的是,标题化合物可以很容易地通过去酯化反应转变为异吲哚并[2,1 - b ]异喹啉-5(7 H)-,这是一种罗塞他汀类似物,经常在各种天然生物碱和合成药物分子中发现。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号