当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Acetic anhydride to the rescue: Facile access to privileged 1,2,3,4-tetrahydropyrazino[1,2-a]indole core via the Castagnoli-Cushman reaction
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-08-28 , DOI: 10.1016/j.tetlet.2018.08.049 Maria Chizhova , Olesya Khoroshilova , Dmitry Dar'in , Mikhail Krasavin
中文翻译:

抢救醋酸酐:通过Castagnoli-Cushman反应轻松获得特权的1,2,3,4-四氢吡嗪并[1,2- a ]吲哚核
更新日期:2018-08-28
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-08-28 , DOI: 10.1016/j.tetlet.2018.08.049 Maria Chizhova , Olesya Khoroshilova , Dmitry Dar'in , Mikhail Krasavin
|
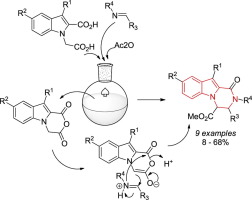
Indole-fused cyclic anhydrides earlier deemed unreactive in the Castagnoli-Cushman reaction with imines have been rendered valid participant in this process. The new reaction format involves the use of respective indole-based dicarboxylic acids and in situ cyclodehydration of the latter by acetic anhydride. This finding validates a fundamentally new approach to synthesizing compounds based on the privileged 1,2,3,4-tetrahydropyrazino[1,2-a]indole core characterized by hitherto undescribed substitution pattern.
中文翻译:

抢救醋酸酐:通过Castagnoli-Cushman反应轻松获得特权的1,2,3,4-四氢吡嗪并[1,2- a ]吲哚核
早先被认为在Castagnoli-Cushman与亚胺的反应中没有反应性的吲哚稠合的环状酸酐已成为该过程的有效参与者。新的反应形式包括使用各自的基于吲哚的二羧酸和后者通过乙酸酐的原位环脱水。这一发现证实了一种根本上新颖的合成化合物的方法,该方法基于特权的1,2,3,4-四氢吡嗪并[1,2- a ]吲哚核,其特征是迄今未描述的取代方式。







































 京公网安备 11010802027423号
京公网安备 11010802027423号