当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper(I)-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides with Fluorinated Imines: The Expanded Scope and Mechanism Insights
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-27 00:00:00 , DOI: 10.1021/acs.joc.8b01743 Liang Wei 1 , Qing-Hua Li 1 , Chun-Jiang Wang 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-27 00:00:00 , DOI: 10.1021/acs.joc.8b01743 Liang Wei 1 , Qing-Hua Li 1 , Chun-Jiang Wang 1, 2
Affiliation
|
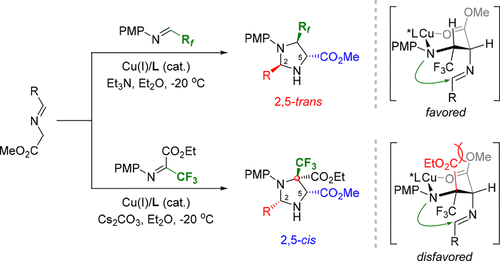
The mechanism of the Cu(I)/(S,Rp)-PPFOMe-catalyzed 1,3-dipolar cycloaddition of azomethine ylides with fluorinated aldimines has been studied using labeling experiments, control experiments, and linear effect experiments, which clearly ruled out the 1,3-DC/epimerization pathways and explained the unusal exo′-selective stereochemistry. This protocol allows for the preparation of a series of highly functionalized fluorinated imidazolidines in good yields with excellent stereoselectivities. Moreover, the current methods have been successfully extended to synthesize more challenging imidazolidines bearing a CF3-containing quaternary stereogenic center via the endo-selective 1,3-DC of azomethine ylides with trifluorinated ketimine under identical reaction conditions.
中文翻译:

铜(I)催化亚胺次膦与氟化亚胺的不对称1,3-偶极环加成反应:扩大的范围和机制的见解
使用标记实验,对照实验和线性效应实验研究了Cu(I)/(S,R p)-PPFOMe催化的1,3-偶极环偶氮亚甲基与氟化亚胺的加成反应的机理,明确排除了1,3- DC /差向异构化途径和解释了此项特殊的外切' -选择性的立体化学。该方案允许以良好的产率和优异的立体选择性制备一系列高度官能化的氟化咪唑烷。而且,目前的方法已经成功地扩展到通过内含物合成具有挑战性的,带有CF 3的季立构中心的咪唑烷类化合物。在相同的反应条件下,用三氟化酮亚胺对甲亚胺烷基化物进行1,3-DC选择性合成。
更新日期:2018-08-27
中文翻译:

铜(I)催化亚胺次膦与氟化亚胺的不对称1,3-偶极环加成反应:扩大的范围和机制的见解
使用标记实验,对照实验和线性效应实验研究了Cu(I)/(S,R p)-PPFOMe催化的1,3-偶极环偶氮亚甲基与氟化亚胺的加成反应的机理,明确排除了1,3- DC /差向异构化途径和解释了此项特殊的外切' -选择性的立体化学。该方案允许以良好的产率和优异的立体选择性制备一系列高度官能化的氟化咪唑烷。而且,目前的方法已经成功地扩展到通过内含物合成具有挑战性的,带有CF 3的季立构中心的咪唑烷类化合物。在相同的反应条件下,用三氟化酮亚胺对甲亚胺烷基化物进行1,3-DC选择性合成。































 京公网安备 11010802027423号
京公网安备 11010802027423号