当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and pharmacology of new psychoactive substance 5F‐CUMYL‐P7AICA, a scaffold‐ hopping analog of synthetic cannabinoid receptor agonists 5F‐CUMYL‐PICA and 5F‐CUMYL‐PINACA
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2018-10-01 , DOI: 10.1002/dta.2491 Samuel D. Banister 1 , Axel Adams 2 , Richard C. Kevin 3 , Christa Macdonald 4 , Michelle Glass 4 , Rochelle Boyd 5 , Mark Connor 5 , Iain S. McGregor 3 , Christopher M. Havel 6 , Stephen J. Bright 7, 8 , Mireia Ventura Vilamala 9 , Cristina Gil Lladanosa 9 , Monica J. Barratt 8, 10, 11 , Roy R. Gerona 2
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2018-10-01 , DOI: 10.1002/dta.2491 Samuel D. Banister 1 , Axel Adams 2 , Richard C. Kevin 3 , Christa Macdonald 4 , Michelle Glass 4 , Rochelle Boyd 5 , Mark Connor 5 , Iain S. McGregor 3 , Christopher M. Havel 6 , Stephen J. Bright 7, 8 , Mireia Ventura Vilamala 9 , Cristina Gil Lladanosa 9 , Monica J. Barratt 8, 10, 11 , Roy R. Gerona 2
Affiliation
|
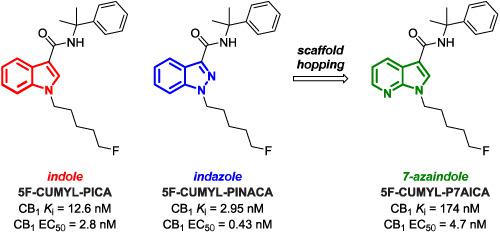
Synthetic cannabinoid receptor agonists (SCRAs) are a dynamic class of new psychoactive substances (NPS), with novel chemotypes emerging each year. Following the putative detection of 5F‐CUMYL‐P7AICA in Australia in 2016, the scaffold‐hopping SCRAs 5F‐CUMYL‐PICA, 5F‐CUMYL‐PINACA, and 5F‐CUMYL‐P7AICA were synthesized and characterized by nuclear magnetic resonance (NMR) spectroscopy, gas chromatography–mass spectrometry (GC–MS), and liquid chromatography–quadrupole time‐of‐flight–MS (LC–QTOF–MS). Since little is known of the pharmacology of 7‐azaindole SCRAs like 5F‐CUMYL‐P7AICA, the binding affinities and functional activities of all compounds at cannabinoid type 1 and type 2 receptors (CB1 and CB2, respectively) were assessed using tritiated radioligand competition experiments and fluorescence‐based plate reader membrane potential assays. Despite CB1 binding affinities differing by over two orders of magnitude (Ki = 2.95–174 nM), all compounds were potent and efficacious CB1 agonists (EC50 = 0.43–4.7 nM), with consistent rank order for binding and functional activity (5F‐CUMYL‐PINACA >5F‐CUMYL‐PICA >5F‐CUMYL‐P7AICA). Additionally, 5F‐CUMYL‐P7AICA was found to exert potent cannabimimetic effects in mice, inducing hypothermia (6°C, 3 mg/kg) through a CB1‐dependent mechanism.
中文翻译:

新的精神活性物质5F‐CUMYL‐P7AICA的合成和药理作用,这是合成大麻素受体激动剂5F‐CUMYL‐PICA和5F‐CUMYL‐PINACA的支架跳跃类似物
合成大麻素受体激动剂(SCRA)是新的精神活性物质(NPS)的动态类别,并且每年都会出现新的化学型。继2016年在澳大利亚推定检测到5F-CUMYL-P7AICA之后,合成了通过脚手架跳动的SCRA 5F-CUMYL-PICA,5F-CUMYL-PINACA和5F-CUMYL-P7AICA并通过核磁共振(NMR)光谱进行了表征,气相色谱-质谱(GC-MS)和液相色谱-四极杆飞行时间-MS(LC-QTOF-MS)。由于对7-氮杂吲哚SCRA(如5F-CUMYL-P7AICA)的药理学知之甚少,因此所有化合物在1型和2型大麻素受体(CB 1和CB 2)上的结合亲和力和功能活性,分别)使用tri化的放射性配体竞争实验和基于荧光的读板器膜电位测定法进行了评估。尽管CB 1的结合亲和力相差两个数量级以上(K i = 2.95–174 nM),但所有化合物均为有效的CB 1激动剂(EC 50 = 0.43–4.7 nM),结合和功能活性的等级顺序一致(5F-CUMYL-PINACA> 5F-CUMYL-PICA> 5F-CUMYL-P7AICA)。此外,还发现5F-CUMYL-P7AICA在小鼠中具有强效的拟大麻作用,通过CB 1依赖性机制诱导体温过低(6°C,3 mg / kg)。
更新日期:2018-10-01
中文翻译:

新的精神活性物质5F‐CUMYL‐P7AICA的合成和药理作用,这是合成大麻素受体激动剂5F‐CUMYL‐PICA和5F‐CUMYL‐PINACA的支架跳跃类似物
合成大麻素受体激动剂(SCRA)是新的精神活性物质(NPS)的动态类别,并且每年都会出现新的化学型。继2016年在澳大利亚推定检测到5F-CUMYL-P7AICA之后,合成了通过脚手架跳动的SCRA 5F-CUMYL-PICA,5F-CUMYL-PINACA和5F-CUMYL-P7AICA并通过核磁共振(NMR)光谱进行了表征,气相色谱-质谱(GC-MS)和液相色谱-四极杆飞行时间-MS(LC-QTOF-MS)。由于对7-氮杂吲哚SCRA(如5F-CUMYL-P7AICA)的药理学知之甚少,因此所有化合物在1型和2型大麻素受体(CB 1和CB 2)上的结合亲和力和功能活性,分别)使用tri化的放射性配体竞争实验和基于荧光的读板器膜电位测定法进行了评估。尽管CB 1的结合亲和力相差两个数量级以上(K i = 2.95–174 nM),但所有化合物均为有效的CB 1激动剂(EC 50 = 0.43–4.7 nM),结合和功能活性的等级顺序一致(5F-CUMYL-PINACA> 5F-CUMYL-PICA> 5F-CUMYL-P7AICA)。此外,还发现5F-CUMYL-P7AICA在小鼠中具有强效的拟大麻作用,通过CB 1依赖性机制诱导体温过低(6°C,3 mg / kg)。










































 京公网安备 11010802027423号
京公网安备 11010802027423号