Insect Biochemistry and Molecular Biology ( IF 3.2 ) Pub Date : 2018-08-26 , DOI: 10.1016/j.ibmb.2018.08.004 Roswitha A Aumann 1 , Marc F Schetelig 2 , Irina Häcker 2
|
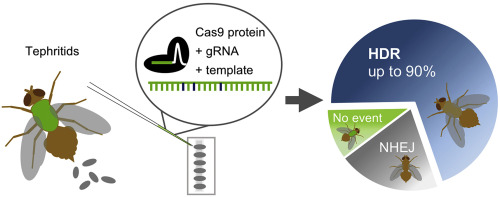
The Mediterranean fruit fly Ceratitis capitata is a highly polyphagous and invasive insect pest, causing enormous economic damage in horticultural systems. A successful and environment-friendly control strategy is the sterile insect technique (SIT) that reduces pest populations through infertile matings with mass-released, sterilized insects. However, the SIT is not readily applicable to each pest species. While transgenic approaches hold great promise to improve critical aspects of the SIT to transfer it to new species, they are suspect to strict or even prohibitive legislation regarding the release of genetically modified (GM) organisms. In contrast, specific mutations created via CRISPR-Cas genome editing are not regulated as GM in the US, and might thus allow creating optimal strains for SIT. Here, we describe highly efficient homology-directed repair genome editing in C. capitata by injecting pre-assembled CRISPR-Cas9 ribonucleoprotein complexes using different guide RNAs and a short single-stranded oligodeoxynucleotide donor to convert an enhanced green fluorescent protein in C. capitata into a blue fluorescent protein. Six out of seven fertile and individually backcrossed G0 individuals generated 57–90% knock-in rate within their total offspring and 70–96% knock-in rate within their phenotypically mutant offspring. Based on the achieved efficiency, this approach could also be used to introduce mutations which do not produce a screenable phenotype and identify positive mutants with a reasonable workload. Furthermore, CRISPR-Cas HDR would allow to recreate mutations formerly identified in classical mutagenesis screens and to transfer them to related species to establish new (SIT-like) pest control systems. Considering the potential that CRISPR-induced alterations in organisms could be classified as non-GM in additional countries, such new strains could potentially be used for pest control applications without the need to struggle with GMO directives.
中文翻译:

使用 Ceratitis headata 中的 Cas9 蛋白进行同源定向修复进行高效基因组编辑
地中海果蝇Ceratitis headata是一种高度杂食性和入侵性害虫,对园艺系统造成巨大的经济损失。昆虫不育技术(SIT)是一种成功且环境友好的控制策略,它通过与大量释放的不育昆虫进行不育交配来减少害虫数量。然而,昆虫不育技术并不容易适用于每种害虫物种。虽然转基因方法有望改善昆虫不育技术的关键方面,将其转移到新物种,但它们可能会受到有关转基因(GM)生物体释放的严格甚至禁止性立法的怀疑。相比之下,通过 CRISPR-Cas 基因组编辑产生的特定突变在美国不受转基因监管,因此可能允许创建 SIT 的最佳菌株。在这里,我们描述了在C. capata中进行高效同源定向修复基因组编辑,通过使用不同的引导 RNA 和短单链寡脱氧核苷酸供体注射预组装的 CRISPR-Cas9 核糖核蛋白复合物,将C. capata中的增强型绿色荧光蛋白转化为蓝色荧光蛋白。七个可育且单独回交的 G 0个体中的六个在其总后代中产生了 57-90% 的敲入率,在其表型突变后代中产生了 70-96% 的敲入率。基于所达到的效率,该方法还可以用于引入不产生可筛选表型的突变,并以合理的工作量识别阳性突变体。此外,CRISPR-Cas HDR 将允许重新创建以前在经典诱变筛选中发现的突变,并将其转移到相关物种以建立新的(类似昆虫不育技术)害虫控制系统。 考虑到 CRISPR 诱导的生物体改变在其他国家可能被归类为非转基因,此类新菌株有可能用于害虫控制应用,而无需与转基因生物指令作斗争。































 京公网安备 11010802027423号
京公网安备 11010802027423号