当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phototransformation of 2,3-Dibromopropyl-2,4,6-tribromophenyl ether (DPTE) in Natural Waters: Important Roles of Dissolved Organic Matter and Chloride Ion
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-09-06 , DOI: 10.1021/acs.est.8b03258 Ya-nan Zhang 1, 2 , Jieqiong Wang 1 , Jingwen Chen 1 , Chengzhi Zhou 1 , Qing Xie 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-09-06 , DOI: 10.1021/acs.est.8b03258 Ya-nan Zhang 1, 2 , Jieqiong Wang 1 , Jingwen Chen 1 , Chengzhi Zhou 1 , Qing Xie 1
Affiliation
|
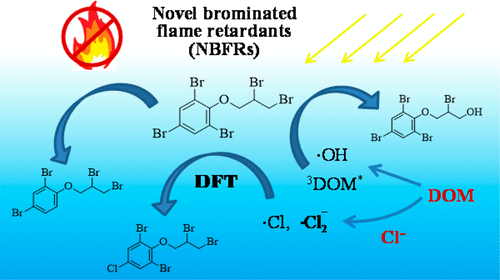
Novel brominated flame retardants (NBFRs) have become ubiquitous emerging organic pollutants. However, little is known about their transformation in natural waters. In this study, aquatic photochemical behavior of a representative NBFR, 2,3-dibromopropyl-2,4,6-tribromophenyl ether (DPTE), was investigated by simulated sunlight irradiation experiment. Results show that DPTE can undergo direct photolysis (apparent quantum yield 0.008 ± 0.001) and hydroxyl radical (•OH) initiated oxidation (second order reaction rate constant 2.4 × 109 M–1·s–1). Dissolved organic matter (DOM) promotes the photodegradation due to generation of excited triplet DOM and •OH. Two chlorinated intermediates were identified in the photodegradation of DPTE in seawaters. Density functional theory calculation showed that •Cl or •Cl2– addition reactions on C–Br sites of the phenyl group and H-abstraction reactions from the propyl group are main reaction pathways of DPTE with the chlorine radicals. The •Cl or •Cl2– addition proceeds via a replacement mechanism to form chlorinated intermediates. Environmental half-lives of DPTE relevant with photodegradation are estimated to be 6.5–1153.9 days in waters of the Yellow River estuarine region. This study provides valuable insights into the phototransformation behavior of DPTE in natural waters, which is helpful for persistence assessment of the NBFRs.
中文翻译:

2,3-二溴丙基-2,4,6-三溴苯基醚(DPTE)在天然水中的光转化:溶解的有机物和氯离子的重要作用
新型溴化阻燃剂(NBFR)已成为无处不在的新兴有机污染物。然而,关于它们在天然水中的转化知之甚少。在这项研究中,通过模拟阳光照射实验研究了代表性的NBFR,2,3-二溴丙基-2,4,6-三溴苯基醚(DPTE)的水生光化学行为。结果表明,DPTE可以进行直接光解(表观量子产率为0.008±0.001),并且羟基自由基(•OH)引发的氧化(二级反应速率常数为2.4×10 9 M –1 ·s –1))。由于产生了激发三重态DOM和•OH,溶解的有机物(DOM)促进了光降解。在海水中DPTE的光降解过程中,发现了两种氯化中间体。密度泛函理论计算表明,•Cl或•氯2 -苯基上的基团的C-溴网站和从丙基H-夺取反应加成反应是与氯自由基DPTE的主要反应途径。•Cl或•Cl 2 –通过替代机制进行加成反应以形成氯化中间体。在黄河河口水域,与光降解有关的DPTE的环境半衰期估计为6.5–1153.9天。这项研究提供了有关DPTE在天然水中的光转化行为的宝贵见解,这有助于对NBFR的持久性进行评估。
更新日期:2018-09-07
中文翻译:

2,3-二溴丙基-2,4,6-三溴苯基醚(DPTE)在天然水中的光转化:溶解的有机物和氯离子的重要作用
新型溴化阻燃剂(NBFR)已成为无处不在的新兴有机污染物。然而,关于它们在天然水中的转化知之甚少。在这项研究中,通过模拟阳光照射实验研究了代表性的NBFR,2,3-二溴丙基-2,4,6-三溴苯基醚(DPTE)的水生光化学行为。结果表明,DPTE可以进行直接光解(表观量子产率为0.008±0.001),并且羟基自由基(•OH)引发的氧化(二级反应速率常数为2.4×10 9 M –1 ·s –1))。由于产生了激发三重态DOM和•OH,溶解的有机物(DOM)促进了光降解。在海水中DPTE的光降解过程中,发现了两种氯化中间体。密度泛函理论计算表明,•Cl或•氯2 -苯基上的基团的C-溴网站和从丙基H-夺取反应加成反应是与氯自由基DPTE的主要反应途径。•Cl或•Cl 2 –通过替代机制进行加成反应以形成氯化中间体。在黄河河口水域,与光降解有关的DPTE的环境半衰期估计为6.5–1153.9天。这项研究提供了有关DPTE在天然水中的光转化行为的宝贵见解,这有助于对NBFR的持久性进行评估。































 京公网安备 11010802027423号
京公网安备 11010802027423号