当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
HN2O2− as a Ligand in Mononuclear Hydrogenhyponitrite‐κ2‐N,O Ruthenium Complexes with Bisphosphane Co‐Ligands
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-10-18 , DOI: 10.1002/chem.201803770 Daniel Beck 1 , Peter Klüfers 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-10-18 , DOI: 10.1002/chem.201803770 Daniel Beck 1 , Peter Klüfers 1
Affiliation
|
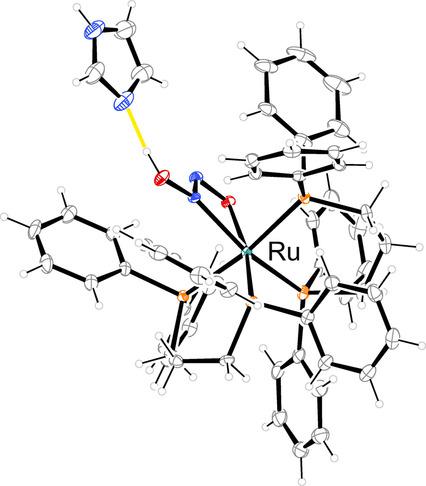
The hyponitrite anion is a tentative intermediate in the reduction of nitric oxide (NO) to nitrous oxide (N2O) catalyzed by nitric‐oxide reductase (NOR) in the process of bacterial denitrification. Owing to the considerable number of known coordination modes for the hyponitrito ligand, its actual bonding form in the enzymatic cycle is a point of current discussion. Here, we contribute to the hardly known ligand properties of a key intermediate, the monoprotonated hyponitrite anion. Three air‐ and water‐stable ruthenium complexes with hydrogenhyponitrite as the ligand were synthesized by using commercially available bisphosphane co‐ligands (1,2‐bis(diphenylphosphino)ethane (dppe), 1,3‐bis(diphenylphosphino)propane (dppp), 1,2‐bis(diphenylphosphino)ethene (dppv)). The starting compounds [Ru(dppe)2(tos)]BF4 (1) and [Ru(dppp)2(tos)]BF4 (2) contained the bidentate coordinating tosylate anion (tos) as a particularly well‐suited leaving group. To confirm the protonated and deprotonated species, X‐ray diffraction, IR, UV/Vis spectroscopy (solution and solid state), solid‐state NMR spectroscopy, and high‐resolution mass spectroscopy were used. DFT calculations give insight into the bonding situation. We report on [Ru(dppe)2(HN2O2)]BF4 (5), [Ru(dppp)2(HN2O2)]BF4 (6), [Ru(dppv)2(HN2O2)]BF4 (7), [Ru(dppp)2(HN2O2)]BF4⋅Imi (9; Imi=imidazole) as the first mononuclear trans‐hydrogenhyponitrite complexes. Isolated deprotonated analogs are [Ru(dppe)2(N2O2)]⋅HImi(BF4) (8) and [Ru(dppv)2(N2O2)] ⋅HImi(BF4)⋅Imi (10).
中文翻译:

HN2O2-作为双核次配体配体的单核氢次锂-κ2-N,O钌配合物中的配体
亚硝酸根阴离子是细菌反硝化过程中一氧化氮还原酶(NOR)催化将一氧化氮(NO)还原为一氧化二氮(N 2 O)的暂定中间体。由于次硝化配体的已知配位方式相当多,其在酶促循环中的实际键合形式是当前讨论的重点。在这里,我们贡献了关键中间体单质子化亚硝酸盐阴离子的鲜为人知的配体性质。通过使用市售的双膦共配体(1,2-双(二苯基膦基)乙烷(dppe),1,3-双(二苯基膦基)丙烷(dppp))合成了三种以氢亚硫酸氢盐为配体的对空气和水稳定的钌络合物1,2-双(二苯基膦基)乙烯(dppv))。起始化合物[Ru(dppe)2(tos)] BF 4(1)和[Ru(dppp)2(tos)] BF 4(2)包含二齿配位甲苯磺酸根阴离子(tos),是一个特别合适的离去基团。为了确认质子化和去质子化的物种,使用了X射线衍射,IR,UV / Vis光谱(溶液和固态),固态NMR光谱和高分辨率质谱。DFT计算可深入了解粘合情况。我们报告了[Ru(dppe)2(HN 2 O 2)] BF 4(5),[Ru(dppp)2(HN 2 O 2)] BF 4(6),的[Ru(dppv)2(HN 2 Ò 2)] BF 4(7),的[Ru(DPPP)2(HN 2 Ò 2)] BF 4 ⋅伊米(9 ;伊米=咪唑)作为第一单核反氢次黄铁矿复合物。分离的去质子化类似物为[Ru(dppe)2(N 2 O 2)] · HImi(BF 4)(8)和[Ru(dppv)2(N 2 O 2)] · HImi(BF 4)·伊美(10)。
更新日期:2018-10-18
中文翻译:

HN2O2-作为双核次配体配体的单核氢次锂-κ2-N,O钌配合物中的配体
亚硝酸根阴离子是细菌反硝化过程中一氧化氮还原酶(NOR)催化将一氧化氮(NO)还原为一氧化二氮(N 2 O)的暂定中间体。由于次硝化配体的已知配位方式相当多,其在酶促循环中的实际键合形式是当前讨论的重点。在这里,我们贡献了关键中间体单质子化亚硝酸盐阴离子的鲜为人知的配体性质。通过使用市售的双膦共配体(1,2-双(二苯基膦基)乙烷(dppe),1,3-双(二苯基膦基)丙烷(dppp))合成了三种以氢亚硫酸氢盐为配体的对空气和水稳定的钌络合物1,2-双(二苯基膦基)乙烯(dppv))。起始化合物[Ru(dppe)2(tos)] BF 4(1)和[Ru(dppp)2(tos)] BF 4(2)包含二齿配位甲苯磺酸根阴离子(tos),是一个特别合适的离去基团。为了确认质子化和去质子化的物种,使用了X射线衍射,IR,UV / Vis光谱(溶液和固态),固态NMR光谱和高分辨率质谱。DFT计算可深入了解粘合情况。我们报告了[Ru(dppe)2(HN 2 O 2)] BF 4(5),[Ru(dppp)2(HN 2 O 2)] BF 4(6),的[Ru(dppv)2(HN 2 Ò 2)] BF 4(7),的[Ru(DPPP)2(HN 2 Ò 2)] BF 4 ⋅伊米(9 ;伊米=咪唑)作为第一单核反氢次黄铁矿复合物。分离的去质子化类似物为[Ru(dppe)2(N 2 O 2)] · HImi(BF 4)(8)和[Ru(dppv)2(N 2 O 2)] · HImi(BF 4)·伊美(10)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号