Redox Biology ( IF 10.7 ) Pub Date : 2018-08-24 , DOI: 10.1016/j.redox.2018.08.017 Marcel Imber , Agnieszka J. Pietrzyk-Brzezinska , Haike Antelmann
|
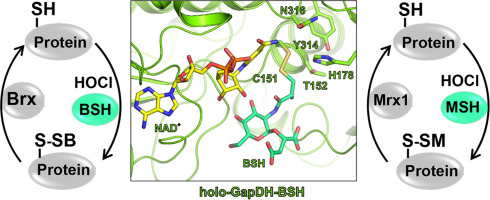
Low molecular weight (LMW) thiols play an important role as thiol-cofactors for many enzymes and are crucial to maintain the reduced state of the cytoplasm. Most Gram-negative bacteria utilize glutathione (GSH) as major LMW thiol. However, in Gram-positive Actinomycetes and Firmicutes alternative LMW thiols, such as mycothiol (MSH) and bacillithiol (BSH) play related roles as GSH surrogates, respectively. Under conditions of hypochlorite stress, MSH and BSH are known to form mixed disulfides with protein thiols, termed as S-mycothiolation or S-bacillithiolation that function in thiol-protection and redox regulation. Protein S-thiolations are widespread redox-modifications discovered in different Gram-positive bacteria, such as Bacillus and Staphylococcus species, Mycobacterium smegmatis, Corynebacterium glutamicum and Corynebacterium diphtheriae. S-thiolated proteins are mainly involved in cellular metabolism, protein translation, redox regulation and antioxidant functions with some conserved targets across bacteria. The reduction of protein S-mycothiolations and S-bacillithiolations requires glutaredoxin-related mycoredoxin and bacilliredoxin pathways to regenerate protein functions.
In this review, we present an overview of the functions of mycothiol and bacillithiol and their physiological roles in protein S-bacillithiolations and S-mycothiolations in Gram-positive bacteria. Significant progress has been made to characterize the role of protein S-thiolation in redox-regulation and thiol protection of main metabolic and antioxidant enzymes. However, the physiological roles of the pathways for regeneration are only beginning to emerge as well as their interactions with other cellular redox systems. Future studies should be also directed to explore the roles of protein S-thiolations and their redox pathways in pathogenic bacteria under infection conditions to discover new drug targets and treatment options against multiple antibiotic resistant bacteria.
中文翻译:

革兰氏阳性菌中可逆蛋白S-硫醇化对氧化还原的调节
低分子量(LMW)硫醇作为许多酶的硫醇辅因子发挥着重要作用,对于维持细胞质的还原状态至关重要。大多数革兰氏阴性细菌利用谷胱甘肽(GSH)作为主要的LMW硫醇。但是,在革兰氏阳性放线菌和Firmicutes中,替代的LMW硫醇,如麦考硫醇(MSH)和杆菌硫醇(BSH),分别作为GSH替代物发挥相关作用。在次氯酸盐胁迫的条件下,已知MSH和BSH与蛋白质硫醇形成混合的二硫键,称为硫醇保护和氧化还原调节功能的S-霉菌硫醇化或S-杆菌硫醇化。蛋白质S硫醇化是在不同的革兰氏阳性细菌中发现的广泛的氧化还原修饰,这些细菌包括芽孢杆菌属和葡萄球菌属,耻垢分枝杆菌,谷氨酸棒状杆菌和白喉棒状杆菌。S-硫醇化蛋白主要参与细胞代谢,蛋白翻译,氧化还原调节和抗氧化功能,并且在细菌中具有一些保守的靶标。蛋白的还原š -mycothiolations和小号-bacillithiolations要求谷相关mycoredoxin和bacilliredoxin途径再生蛋白质的功能。
在这篇综述中,我们概述了霉菌硫醇和杆菌硫醇的功能,以及它们在革兰氏阳性细菌中蛋白质S -bacillithiolations和S -mycothiolations中的生理作用。在表征蛋白S-硫醇化在主要代谢和抗氧化酶的氧化还原调节和硫醇保护中的作用方面已经取得了重大进展。然而,再生途径的生理作用及其与其他细胞氧化还原系统的相互作用才刚刚开始显现。未来的研究也应针对探索蛋白质S的作用感染条件下致病菌中的β-硫醇化及其氧化还原途径,以发现针对多种抗生素抗性细菌的新药靶点和治疗选择。






























 京公网安备 11010802027423号
京公网安备 11010802027423号