当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Shape-Dependent Electrocatalytic Activity of Iridium Oxide Decorated Erbium Pyrosilicate toward the Hydrogen Evolution Reaction over the Entire pH Range
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-08-24 00:00:00 , DOI: 10.1021/acscatal.8b01363 Paramita Karfa 1 , Kartick C. Majhi 1 , Rashmi Madhuri 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-08-24 00:00:00 , DOI: 10.1021/acscatal.8b01363 Paramita Karfa 1 , Kartick C. Majhi 1 , Rashmi Madhuri 1
Affiliation
|
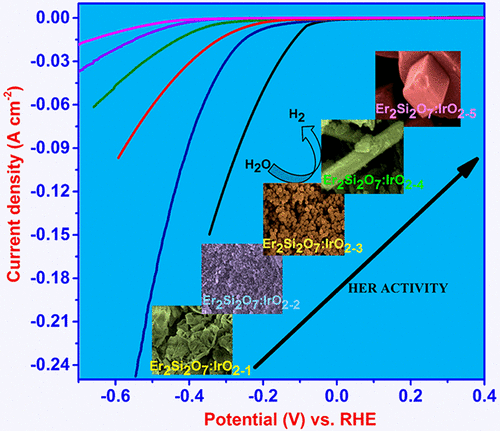
The synthesis of iridium oxide decorated erbium pyrosilicate (Er2Si2O7:IrO2) was performed by a simple sol–gel technique. By simple alteration of the reaction parameters, different shapes (i.e., cubes, rods, large spheres, small spheres, and sheets) of Er2Si2O7:IrO2 were obtained and their catalytic activities were tested toward the hydrogen evolution reaction (HER). Among the different morphologies of Er2Si2O7:IrO2, cube-shaped nanoparticles (Er2Si2O7:IrO2–5) with sharp edges provided promising HER activity over a wide pH range from 0 to 14 in acidic, neutral, and basic media. Er2Si2O7:IrO2–5 exhibited an onset potential of −0.076 V with a very high current density of 252 mA cm–2 (at −0.54 V). The overpotential and Tafel slope for the HER using Er2Si2O7:IrO2–5 were found to be 130 mV and 49 mV/dec, 170 mV and 59 mV/dec, and 190 mV and 67 mV/dec in 0.5 M H2SO4, 1.0 M KOH, and 2.0 M PBS, respectively. The low cost, highly active electrocatalyst shows robust durability over acidic medium for nearly 250 min. Such a superior catalytic activity can be attributed to the synergistic effect between IrO2 and erbium pyrosilicate, in which the 4f orbital (not fully occupied) of rare-earth elements may have occupied the 5d orbital and become valence electrons, resulting in the improved HER activity.
中文翻译:

氧化铱修饰的焦硅酸Er对整个pH范围内氢发生反应的形状依赖性电催化活性
氧化铱修饰的焦硅酸((Er 2 Si 2 O 7:IrO 2)的合成是通过简单的溶胶-凝胶技术进行的。通过简单地改变反应参数,可以获得Er 2 Si 2 O 7:IrO 2的不同形状(即立方体,棒状,大球体,小球体和片状),并测试了它们对制氢反应的催化活性(她)。在Er 2 Si 2 O 7:IrO 2的不同形态中,有立方体形的纳米颗粒(Er 2 Si 2 O 7:IrO 2–5)具有锋利的边缘,在酸性,中性和碱性介质中,在0至14的宽pH范围内,均提供了有希望的HER活性。Er 2 Si 2 O 7:IrO 2–5表现出-0.076 V的启动电位,并且具有252 mA cm –2的非常高的电流密度(在-0.54 V时)。发现使用Er 2 Si 2 O 7:IrO 2–5的HER的过电势和Tafel斜率分别为130 mV和49 mV / dec,170 mV和59 mV / dec,190 mV和67 mV / dec(0.5) MH 2 SO 4,1.0 M KOH和2.0 M PBS。低成本,高活性的电催化剂在酸性介质中显示了近250分钟的坚固耐用性。这种优异的催化活性可归因于IrO 2和焦硅酸之间的协同作用,其中稀土元素的4f轨道(未完全占据)可能已经占据了5d轨道并变成了价电子,从而改善了HER活动。
更新日期:2018-08-24
中文翻译:

氧化铱修饰的焦硅酸Er对整个pH范围内氢发生反应的形状依赖性电催化活性
氧化铱修饰的焦硅酸((Er 2 Si 2 O 7:IrO 2)的合成是通过简单的溶胶-凝胶技术进行的。通过简单地改变反应参数,可以获得Er 2 Si 2 O 7:IrO 2的不同形状(即立方体,棒状,大球体,小球体和片状),并测试了它们对制氢反应的催化活性(她)。在Er 2 Si 2 O 7:IrO 2的不同形态中,有立方体形的纳米颗粒(Er 2 Si 2 O 7:IrO 2–5)具有锋利的边缘,在酸性,中性和碱性介质中,在0至14的宽pH范围内,均提供了有希望的HER活性。Er 2 Si 2 O 7:IrO 2–5表现出-0.076 V的启动电位,并且具有252 mA cm –2的非常高的电流密度(在-0.54 V时)。发现使用Er 2 Si 2 O 7:IrO 2–5的HER的过电势和Tafel斜率分别为130 mV和49 mV / dec,170 mV和59 mV / dec,190 mV和67 mV / dec(0.5) MH 2 SO 4,1.0 M KOH和2.0 M PBS。低成本,高活性的电催化剂在酸性介质中显示了近250分钟的坚固耐用性。这种优异的催化活性可归因于IrO 2和焦硅酸之间的协同作用,其中稀土元素的4f轨道(未完全占据)可能已经占据了5d轨道并变成了价电子,从而改善了HER活动。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号