当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Asciminib (ABL001), an Allosteric Inhibitor of the Tyrosine Kinase Activity of BCR-ABL1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acs.jmedchem.8b01040 Joseph Schoepfer 1 , Wolfgang Jahnke 1 , Giuliano Berellini , Silvia Buonamici , Simona Cotesta 1 , Sandra W. Cowan-Jacob 1 , Stephanie Dodd 2 , Peter Drueckes 1 , Doriano Fabbro , Tobias Gabriel 1 , Jean-Marc Groell 1 , Robert M. Grotzfeld 1 , A. Quamrul Hassan , Chrystèle Henry 1 , Varsha Iyer , Darryl Jones 1 , Franco Lombardo , Alice Loo 2 , Paul W. Manley 1 , Xavier Pellé 1 , Gabriele Rummel 1 , Bahaa Salem 1 , Markus Warmuth , Andrew A. Wylie , Thomas Zoller 1 , Andreas L. Marzinzik 1 , Pascal Furet 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acs.jmedchem.8b01040 Joseph Schoepfer 1 , Wolfgang Jahnke 1 , Giuliano Berellini , Silvia Buonamici , Simona Cotesta 1 , Sandra W. Cowan-Jacob 1 , Stephanie Dodd 2 , Peter Drueckes 1 , Doriano Fabbro , Tobias Gabriel 1 , Jean-Marc Groell 1 , Robert M. Grotzfeld 1 , A. Quamrul Hassan , Chrystèle Henry 1 , Varsha Iyer , Darryl Jones 1 , Franco Lombardo , Alice Loo 2 , Paul W. Manley 1 , Xavier Pellé 1 , Gabriele Rummel 1 , Bahaa Salem 1 , Markus Warmuth , Andrew A. Wylie , Thomas Zoller 1 , Andreas L. Marzinzik 1 , Pascal Furet 1
Affiliation
|
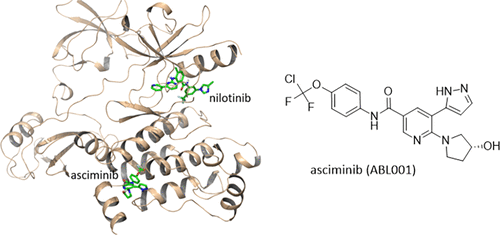
Chronic myelogenous leukemia (CML) arises from the constitutive activity of the BCR-ABL1 oncoprotein. Tyrosine kinase inhibitors (TKIs) that target the ATP-binding site have transformed CML into a chronic manageable disease. However, some patients develop drug resistance due to ATP-site mutations impeding drug binding. We describe the discovery of asciminib (ABL001), the first allosteric BCR-ABL1 inhibitor to reach the clinic. Asciminib binds to the myristate pocket of BCR-ABL1 and maintains activity against TKI-resistant ATP-site mutations. Although resistance can emerge due to myristate-site mutations, these are sensitive to ATP-competitive inhibitors so that combinations of asciminib with ATP-competitive TKIs suppress the emergence of resistance. Fragment-based screening using NMR and X-ray yielded ligands for the myristate pocket. An NMR-based conformational assay guided the transformation of these inactive ligands into ABL1 inhibitors. Further structure-based optimization for potency, physicochemical, pharmacokinetic, and drug-like properties, culminated in asciminib, which is currently undergoing clinical studies in CML patients.
中文翻译:

发现Ascminib(ABL001),BCR-ABL1酪氨酸激酶活性的变构抑制剂
慢性粒细胞性白血病(CML)源自BCR-ABL1癌蛋白的组成型活性。靶向ATP结合位点的酪氨酸激酶抑制剂(TKIs)已将CML转化为慢性可控制的疾病。但是,由于ATP位点突变阻碍了药物结合,一些患者出现了耐药性。我们描述了asciminib(ABL001)的发现,这是第一种到达临床的变构BCR-ABL1抑制剂。Asciminib结合BCR-ABL1的肉豆蔻口袋,并保持抗TKI耐药性ATP站点突变的活性。尽管由于肉豆蔻酸位点突变会产生抗药性,但这些突变对ATP竞争性抑制剂敏感,因此,asciminib与ATP竞争性TKI的组合可抑制抗药性的出现。使用NMR和X射线的基于片段的筛选产生了肉豆蔻口袋的配体。基于NMR的构象分析指导了这些非活性配体向ABL1抑制剂的转化。对效力,理化,药代动力学和类药物特性进行进一步的基于结构的优化,最终达到了asciminib的要求,目前正在对CML患者进行临床研究。
更新日期:2018-08-23
中文翻译:

发现Ascminib(ABL001),BCR-ABL1酪氨酸激酶活性的变构抑制剂
慢性粒细胞性白血病(CML)源自BCR-ABL1癌蛋白的组成型活性。靶向ATP结合位点的酪氨酸激酶抑制剂(TKIs)已将CML转化为慢性可控制的疾病。但是,由于ATP位点突变阻碍了药物结合,一些患者出现了耐药性。我们描述了asciminib(ABL001)的发现,这是第一种到达临床的变构BCR-ABL1抑制剂。Asciminib结合BCR-ABL1的肉豆蔻口袋,并保持抗TKI耐药性ATP站点突变的活性。尽管由于肉豆蔻酸位点突变会产生抗药性,但这些突变对ATP竞争性抑制剂敏感,因此,asciminib与ATP竞争性TKI的组合可抑制抗药性的出现。使用NMR和X射线的基于片段的筛选产生了肉豆蔻口袋的配体。基于NMR的构象分析指导了这些非活性配体向ABL1抑制剂的转化。对效力,理化,药代动力学和类药物特性进行进一步的基于结构的优化,最终达到了asciminib的要求,目前正在对CML患者进行临床研究。

































 京公网安备 11010802027423号
京公网安备 11010802027423号