当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ammonia Synthesis from Electrocatalytic N2 Reduction under Ambient Conditions by Fe2O3 Nanorods
ChemCatChem ( IF 3.8 ) Pub Date : 2018-09-11 , DOI: 10.1002/cctc.201801208 Xiaojiao Xiang 1, 2 , Zao Wang 2 , Xifeng Shi 3 , Meikun Fan 1 , Xuping Sun 2
ChemCatChem ( IF 3.8 ) Pub Date : 2018-09-11 , DOI: 10.1002/cctc.201801208 Xiaojiao Xiang 1, 2 , Zao Wang 2 , Xifeng Shi 3 , Meikun Fan 1 , Xuping Sun 2
Affiliation
|
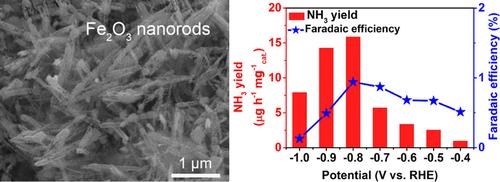
The conventional Haber‐Bosch process for industrial ammonia production from N2 and H2 is not only energy‐intensive but also releases a large amount of CO2. The electrocatalytic nitrogen reduction reaction (NRR) is regarded as a sustainable and environmentally‐benign alternative approach for NH3 production under ambient conditions. In this communication, it is reported that Fe2O3 nanorods act as an efficient electrocatalyst for the NRR. In 0.1 M Na2SO4, it attains a Faradic efficiency of 0.94 % and NH3 yield of 15.9 μg h−1 mg−1cat. at −0.8 V vs. reversible hydrogen electrode. Furthermore, this catalyst also shows good stability during electrolysis and recycling tests.
中文翻译:

Fe2O3纳米棒在环境条件下电催化还原N2合成氨
用于从N 2和H 2生产工业氨的常规Haber-Bosch工艺不仅耗能大,而且还会释放大量CO 2。电催化氮还原反应(NRR)被认为是在环境条件下生产NH 3的一种可持续且对环境无害的替代方法。据报道,Fe 2 O 3纳米棒可作为NRR的有效电催化剂。在0.1 M Na 2 SO 4中,法拉第效率为0.94%,NH 3产率为15.9μgh -1 mg -1 cat。在-0.8 V相对于可逆氢电极的情况下。此外,该催化剂在电解和循环测试中也显示出良好的稳定性。
更新日期:2018-09-11
中文翻译:

Fe2O3纳米棒在环境条件下电催化还原N2合成氨
用于从N 2和H 2生产工业氨的常规Haber-Bosch工艺不仅耗能大,而且还会释放大量CO 2。电催化氮还原反应(NRR)被认为是在环境条件下生产NH 3的一种可持续且对环境无害的替代方法。据报道,Fe 2 O 3纳米棒可作为NRR的有效电催化剂。在0.1 M Na 2 SO 4中,法拉第效率为0.94%,NH 3产率为15.9μgh -1 mg -1 cat。在-0.8 V相对于可逆氢电极的情况下。此外,该催化剂在电解和循环测试中也显示出良好的稳定性。































 京公网安备 11010802027423号
京公网安备 11010802027423号