当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bigger is Surprisingly Better: Agglomerates of Larger RuP Nanoparticles Outperform Benchmark Pt Nanocatalysts for the Hydrogen Evolution Reaction
Advanced Materials ( IF 27.4 ) Pub Date : 2018-08-21 , DOI: 10.1002/adma.201800047
Jie Yu 1 , Yanan Guo 1 , Sixuan She 1 , Shuanshuan Miao 1 , Meng Ni 2 , Wei Zhou 1 , Meilin Liu 3 , Zongping Shao 1, 4
Advanced Materials ( IF 27.4 ) Pub Date : 2018-08-21 , DOI: 10.1002/adma.201800047
Jie Yu 1 , Yanan Guo 1 , Sixuan She 1 , Shuanshuan Miao 1 , Meng Ni 2 , Wei Zhou 1 , Meilin Liu 3 , Zongping Shao 1, 4
Affiliation
|
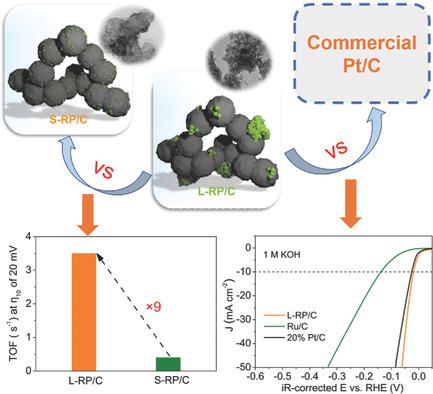
Although metallic ruthenium (Ru) is a potential electrocatalyst for the hydrogen evolution reaction (HER) to replace platinum (Pt) at a cost of only ≈4% of Pt, the persistent dissolution of Ru under operation conditions remains a challenge. Here, it is reported that agglomerates of large ruthenium phosphide (RuP) particles (L‐RP, ≈32 nm) show outstanding HER performance in pH‐universal electrolytes, which particularly demonstrates a surprisingly higher intrinsic activity and durability than small nanoparticles of RuP (S‐RP, ≈3 nm) or metallic Ru on carbon supports. This is especially true in basic media, achieving electrocatalytic activity comparable to or even outperforming that of Pt/C, as reflected by lower overpotential at 10 mA cm−2, smaller Tafel slope, larger exchange current density, and higher turnover frequency while maintaining 200 h stable operation. Calculations suggest that ΔGH* of RuP is much closer to zero than that of metallic Ru, and phosphorous doping is proven to enhance the rate of proton transfer in HER, contributing in part to the improved activity of RuP. The better performance of L‐RP than that of S‐RP is ascribed largely to the stabilization of the P species due to the lowered surface energy of large particles. Furthermore, the relatively low‐cost materials and facile synthesis make L‐RP/C a highly attractive next‐generation HER electrocatalyst.
中文翻译:

更大更好地令人惊讶:较大的RuP纳米颗粒的团聚体在放氢反应方面的性能优于基准Pt纳米催化剂
尽管金属钌(Ru)是潜在的放氢反应(HER)代替铂(Pt)的电催化剂,其成本仅为Pt的约4%,但在操作条件下Ru的持续溶解仍然是一个挑战。在这里,据报道,大的磷化钌(RuP)颗粒(L-RP,≈32nm)的团聚体在pH通用型电解质中显示出出色的HER性能,特别是与小粒径的RuP纳米颗粒相比,其内在活性和耐久性令人惊讶地更高( S‐RP,≈3nm)或碳载体上的金属Ru。在碱性介质中尤其如此,其电催化活性可与Pt / C相比甚至更高,如10 mA cm -2处较低的过电势所反映,较小的塔菲尔斜率,较大的交换电流密度和较高的周转频率,同时保持200小时的稳定运行。计算表明,RuP的ΔG H *比金属Ru的ΔG H *更接近零,并且磷掺杂已被证明可以提高HER中质子转移的速率,部分有助于RuP活性的提高。L-RP的性能优于S-RP,这在很大程度上归因于P物种的稳定,这是因为大颗粒的表面能降低了。此外,相对低成本的材料和便捷的合成方法使L‐RP / C成为下一代HER电催化剂的极具吸引力。
更新日期:2018-08-21
中文翻译:

更大更好地令人惊讶:较大的RuP纳米颗粒的团聚体在放氢反应方面的性能优于基准Pt纳米催化剂
尽管金属钌(Ru)是潜在的放氢反应(HER)代替铂(Pt)的电催化剂,其成本仅为Pt的约4%,但在操作条件下Ru的持续溶解仍然是一个挑战。在这里,据报道,大的磷化钌(RuP)颗粒(L-RP,≈32nm)的团聚体在pH通用型电解质中显示出出色的HER性能,特别是与小粒径的RuP纳米颗粒相比,其内在活性和耐久性令人惊讶地更高( S‐RP,≈3nm)或碳载体上的金属Ru。在碱性介质中尤其如此,其电催化活性可与Pt / C相比甚至更高,如10 mA cm -2处较低的过电势所反映,较小的塔菲尔斜率,较大的交换电流密度和较高的周转频率,同时保持200小时的稳定运行。计算表明,RuP的ΔG H *比金属Ru的ΔG H *更接近零,并且磷掺杂已被证明可以提高HER中质子转移的速率,部分有助于RuP活性的提高。L-RP的性能优于S-RP,这在很大程度上归因于P物种的稳定,这是因为大颗粒的表面能降低了。此外,相对低成本的材料和便捷的合成方法使L‐RP / C成为下一代HER电催化剂的极具吸引力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号