当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluating Nucleophile Byproduct Formation during Phosphine- and Amine-Promoted Thiol–Methyl Acrylate Reactions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-22 00:00:00 , DOI: 10.1021/acs.joc.8b01471 Stephen H. Frayne 1 , Brian H. Northrop 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-08-22 00:00:00 , DOI: 10.1021/acs.joc.8b01471 Stephen H. Frayne 1 , Brian H. Northrop 1
Affiliation
|
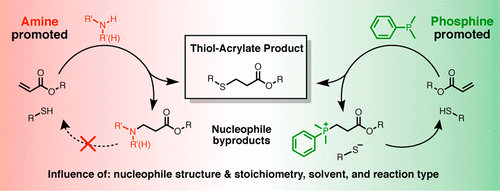
The commonly accepted mechanism of nucleophile-initiated thiol–acrylate reactions requires the formation of undesired nucleophile byproducts. A systematic evaluation of the formation of such nucleophile byproducts has been carried out to understand the relationships between byproduct formation and nucleophile structure, stoichiometry, solvent, and reaction type. Three common nucleophiles for thiol-Michael reactions were investigated: dimethylphenylphosphine (DMPP), diethylamine (DEA), and hexylamine (HA). The formation of phosphonium ester and aza-Michael byproducts upon initiating a representative thiol–acrylate reaction between 1-hexanethiol and methyl acrylate at a range of initiator loading (0.01–10.0 equiv) and in different solvents (neat, DMSO, THF, and CHCl3) was determined by 1H NMR spectroscopy. The influence of reaction type was investigated by expanding from small molecule reactions to end group thiol–acrylate functionalization of PEG-diacrylate polymers and through investigations of polymer–polymer coupling reactions. Results indicate that the propensity of forming nucleophile byproducts varies with nucleophile type, solvent, and reaction type. Interestingly, for all but polymer–polymer ligation reactions, nucleophile byproduct formation is largely unobserved for nitrogen-centered nucleophiles DEA and HA and essentially nonexistent for the phorphorous-centered nucleophile DMPP. A rationale for the differences in nucleophile byproduct formation for DMPP, DEA, and HA is proposed and supported by experimental and computational analysis.
中文翻译:

在膦和胺促进的硫醇-丙烯酸甲酯反应过程中评估亲核副产物的形成
亲核试剂引发的巯基丙烯酸酯反应的普遍接受的机制要求形成不期望的亲核试剂副产物。已经对这类亲核试剂副产物的形成进行了系统评价,以了解副产物形成与亲核试剂结构,化学计量,溶剂和反应类型之间的关系。研究了硫醇-迈克尔反应的三种常见亲核试剂:二甲基苯基膦(DMPP),二乙胺(DEA)和己胺(HA)。在引发剂负载量(0.01-10.0当量)和不同溶剂(纯溶剂,DMSO,THF和CHCl)中,在1-己硫醇和丙烯酸甲酯之间引发代表性的硫醇-丙烯酸酯反应时,形成ester酯和氮杂-Michael副产物3)由1确定1 H NMR光谱。通过从小分子反应扩展到PEG-二丙烯酸酯聚合物的端基巯基丙烯酸酯官能化以及对聚合物-聚合物偶联反应的研究,研究了反应类型的影响。结果表明,亲核试剂副产物形成的倾向随亲核试剂类型,溶剂和反应类型的不同而不同。有趣的是,对于除聚合物-聚合物连接反应以外的所有反应,亲核副产物的形成在以氮为中心的亲核体DEA和HA中几乎没有观察到,而以磷为中心的亲核体DMPP则基本上不存在。提出了DMPP,DEA和HA亲核副产物形成差异的基本原理,并得到了实验和计算分析的支持。
更新日期:2018-08-22
中文翻译:

在膦和胺促进的硫醇-丙烯酸甲酯反应过程中评估亲核副产物的形成
亲核试剂引发的巯基丙烯酸酯反应的普遍接受的机制要求形成不期望的亲核试剂副产物。已经对这类亲核试剂副产物的形成进行了系统评价,以了解副产物形成与亲核试剂结构,化学计量,溶剂和反应类型之间的关系。研究了硫醇-迈克尔反应的三种常见亲核试剂:二甲基苯基膦(DMPP),二乙胺(DEA)和己胺(HA)。在引发剂负载量(0.01-10.0当量)和不同溶剂(纯溶剂,DMSO,THF和CHCl)中,在1-己硫醇和丙烯酸甲酯之间引发代表性的硫醇-丙烯酸酯反应时,形成ester酯和氮杂-Michael副产物3)由1确定1 H NMR光谱。通过从小分子反应扩展到PEG-二丙烯酸酯聚合物的端基巯基丙烯酸酯官能化以及对聚合物-聚合物偶联反应的研究,研究了反应类型的影响。结果表明,亲核试剂副产物形成的倾向随亲核试剂类型,溶剂和反应类型的不同而不同。有趣的是,对于除聚合物-聚合物连接反应以外的所有反应,亲核副产物的形成在以氮为中心的亲核体DEA和HA中几乎没有观察到,而以磷为中心的亲核体DMPP则基本上不存在。提出了DMPP,DEA和HA亲核副产物形成差异的基本原理,并得到了实验和计算分析的支持。















































 京公网安备 11010802027423号
京公网安备 11010802027423号