Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A链一级结构差异对胰岛素片段聚集的影响
ACS Omega ( IF 3.7 ) Pub Date : 2018-08-21 00:00:00 , DOI: 10.1021/acsomega.8b00500 Paul P Nakka 1 , Ke Li 1 , Daniel Forciniti 1
Affiliation
|
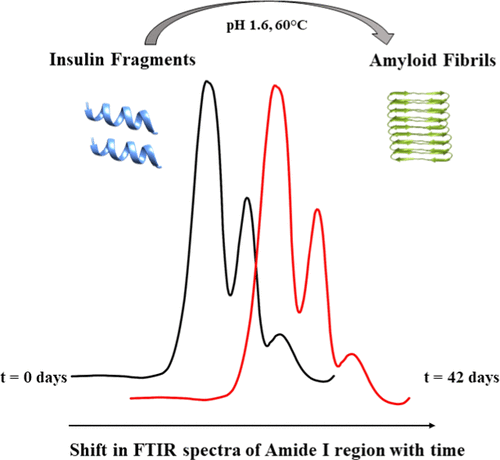
牛胰岛素和人胰岛素具有相似的一级结构。在本文中,对牛胰岛素和人胰岛素的胰岛素 A 链区域的氨基酸组成不同进行了研究。在固相肽合成中合成了牛胰岛素片段(BIF)和人胰岛素片段(HIF)。使用分数阶乘分辨率 III 实验设计研究了 pH、温度、尿素、离子强度和搅拌对原纤维形成的影响。通过荧光和红外光谱以及光学显微镜监测纤维颤动。两种片段在 pH 1.6 和 60 °C 的温度下均形成原纤维。使用二参数动力学模型确定滞后时间和表观聚集生长速率常数。结果发现,牛胰岛素片段的滞后时间比人胰岛素片段短,而 HIF 的指数期速率比 BIF 更快。在两个片段中都观察到β-折叠含量随时间增加。在β-折叠增加之前,α-螺旋最初减少,随后在从滞后期到伸长期的过渡期间中间增加。温度和离子强度是滞后阶段最重要的实验因素之一,而离子强度在两个片段的延伸阶段都被 pH 值取代。刚果红结合证实了富含反平行β-折叠的环状低聚物结构的存在,其倾向于形成富含平行β-折叠的原纤维。

"点击查看英文标题和摘要"





















































 京公网安备 11010802027423号
京公网安备 11010802027423号