当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Activation of Human Caseinolytic Protease P (ClpP)
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-09-19 , DOI: 10.1002/anie.201808189 Matthias Stahl 1, 2 , Vadim S. Korotkov 1 , Dóra Balogh 1 , Leonhard M. Kick 1 , Malte Gersch 1, 3 , Axel Pahl 1, 4 , Pavel Kielkowski 1 , Klaus Richter 1 , Sabine Schneider 1 , Stephan A. Sieber 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-09-19 , DOI: 10.1002/anie.201808189 Matthias Stahl 1, 2 , Vadim S. Korotkov 1 , Dóra Balogh 1 , Leonhard M. Kick 1 , Malte Gersch 1, 3 , Axel Pahl 1, 4 , Pavel Kielkowski 1 , Klaus Richter 1 , Sabine Schneider 1 , Stephan A. Sieber 1
Affiliation
|
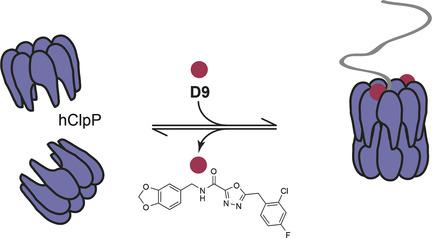
Caseinolytic protease P (ClpP) is the proteolytic component of the ClpXP protein degradation complex. Eukaryotic ClpP was recently found to act within the mitochondria‐specific unfolded protein response (UPRmt). However, its detailed function and dedicated regulation remain largely unexplored. A small molecule (D9) acts as a potent and species‐selective activator of human ClpP (hClpP) by mimicking the natural chaperone ClpX. Structure–activity relationship studies highlight the importance of a halogenated benzyl motif within D9 that interacts with a unique aromatic amino acid network in hClpP. Mutational and structural studies suggest that this YYW motif tightly controls hClpP activity and regulates substrate turnover by interaction with cognate ligands. This signature motif is unique to ClpP from higher organisms and does not exist in tested bacterial homologues, allowing a species‐selective analysis. Thus, D9 is a versatile tool to analyze mechanistic features of hClpP.
中文翻译:

人类酪蛋白水解蛋白酶P(ClpP)的选择性激活
酪蛋白水解蛋白酶P(ClpP)是ClpXP蛋白降解复合物的蛋白水解成分。最近发现真核ClpP在线粒体特异性未折叠蛋白反应(UPR mt)中起作用。但是,它的详细功能和专门的法规仍未开发。一个小分子(D9)通过模仿天然伴侣ClpX,充当人类ClpP(hClpP)的一种有效的物种选择性激活剂。结构-活性关系研究突出了D9中卤化苄基基序的重要性与hClpP中独特的芳香氨基酸网络相互作用。突变和结构研究表明,该YYW基序通过与同源配体的相互作用紧密控制hClpP活性并调节底物更新。此特征性基序是高等生物的ClpP所特有的,并且在经过测试的细菌同源物中不存在,因此可以进行物种选择性分析。因此,D9是分析hClpP力学特征的通用工具。
更新日期:2018-09-19
中文翻译:

人类酪蛋白水解蛋白酶P(ClpP)的选择性激活
酪蛋白水解蛋白酶P(ClpP)是ClpXP蛋白降解复合物的蛋白水解成分。最近发现真核ClpP在线粒体特异性未折叠蛋白反应(UPR mt)中起作用。但是,它的详细功能和专门的法规仍未开发。一个小分子(D9)通过模仿天然伴侣ClpX,充当人类ClpP(hClpP)的一种有效的物种选择性激活剂。结构-活性关系研究突出了D9中卤化苄基基序的重要性与hClpP中独特的芳香氨基酸网络相互作用。突变和结构研究表明,该YYW基序通过与同源配体的相互作用紧密控制hClpP活性并调节底物更新。此特征性基序是高等生物的ClpP所特有的,并且在经过测试的细菌同源物中不存在,因此可以进行物种选择性分析。因此,D9是分析hClpP力学特征的通用工具。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号