当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cooperative Catalysis by Surface Lewis Acid/Silanol for Selective Fructose Etherification on Sn-SPP Zeolite
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-08-20 00:00:00 , DOI: 10.1021/acscatal.8b01615 Tyler R. Josephson 1, 2, 3 , Robert F. DeJaco 3 , Swagata Pahari 2 , Limin Ren 3 , Qiang Guo 3 , Michael Tsapatsis 3 , J. Ilja Siepmann 2, 3 , Dionisios G. Vlachos 1 , Stavros Caratzoulas 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-08-20 00:00:00 , DOI: 10.1021/acscatal.8b01615 Tyler R. Josephson 1, 2, 3 , Robert F. DeJaco 3 , Swagata Pahari 2 , Limin Ren 3 , Qiang Guo 3 , Michael Tsapatsis 3 , J. Ilja Siepmann 2, 3 , Dionisios G. Vlachos 1 , Stavros Caratzoulas 1
Affiliation
|
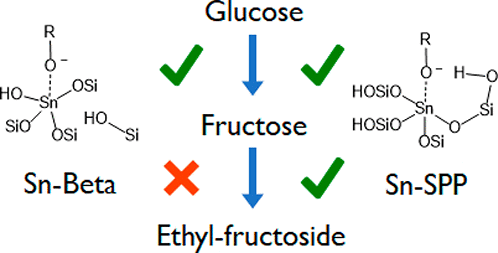
While Lewis-acid zeolites, such as Sn-Beta, catalyze glucose isomerization in an alcoholic medium, mesoporous Sn-SPP catalyzes both glucose isomerization to fructose and fructose etherification (formally ketalization) to ethyl fructoside, enabling fructose yields in excess of the glucose/fructose equilibrium. Using periodic density functional theory calculations and force-field-based Monte Carlo simulations, the ketalization reaction mechanism and adsorption behavior were examined. The silanols on the Sn-SPP mesopore surface facilitate the ketalization reaction through hydrogen bonding interactions at the transition state, only possible via a Sn–O–Si−OH moiety, present in Sn-SPP but not in Sn-Beta. Fructose ketalization is favored over glucose acetalization due to differences in stability of the oxonium intermediates, which are stabilized by the Sn-SPP active site. The open site of hydrophobic Sn-Beta cannot perform these reactions because its active site does not contain an adjacent silanol of the right geometry. In addition to the more favorable activation barrier of the catalytic process, the adsorption at the catalytic site in Sn-SPP is also found to be more favorable than for Sn-Beta, in spite of competitive adsorption between fructose and ethanol in the ethanol-saturated zeolites.
中文翻译:

表面路易斯酸/硅烷醇协同催化在Sn-SPP沸石上选择性果糖醚化
路易斯酸沸石(例如Sn-β)在酒精介质中催化葡萄糖异构化,而介孔Sn-SPP既催化葡萄糖异构化为果糖,又催化果糖醚化(正式缩酮化)为乙基果糖苷,使果糖收率超过葡萄糖/果糖平衡。使用周期性密度泛函理论计算和基于力场的蒙特卡洛模拟,研究了缩酮化反应机理和吸附行为。Sn-SPP中孔表面上的硅烷醇通过过渡态的氢键相互作用促进了缩酮化反应,只有通过Sn-SPP中存在的Sn-O-Si-OH部分才能存在,而Sn-Beta中不存在。由于氧中间体的稳定性不同,果糖缩酮化优于葡萄糖缩醛化,通过Sn-SPP活性位点稳定。疏水性Sn-Beta的开放位点不能进行这些反应,因为其活性位点不包含几何形状合适的相邻硅烷醇。除了催化过程中更有利的活化障碍外,尽管果糖和乙醇在饱和乙醇中竞争性吸附,但发现Sn-SPP中催化部位的吸附也比Sn-Beta更为有利。沸石。
更新日期:2018-08-20
中文翻译:

表面路易斯酸/硅烷醇协同催化在Sn-SPP沸石上选择性果糖醚化
路易斯酸沸石(例如Sn-β)在酒精介质中催化葡萄糖异构化,而介孔Sn-SPP既催化葡萄糖异构化为果糖,又催化果糖醚化(正式缩酮化)为乙基果糖苷,使果糖收率超过葡萄糖/果糖平衡。使用周期性密度泛函理论计算和基于力场的蒙特卡洛模拟,研究了缩酮化反应机理和吸附行为。Sn-SPP中孔表面上的硅烷醇通过过渡态的氢键相互作用促进了缩酮化反应,只有通过Sn-SPP中存在的Sn-O-Si-OH部分才能存在,而Sn-Beta中不存在。由于氧中间体的稳定性不同,果糖缩酮化优于葡萄糖缩醛化,通过Sn-SPP活性位点稳定。疏水性Sn-Beta的开放位点不能进行这些反应,因为其活性位点不包含几何形状合适的相邻硅烷醇。除了催化过程中更有利的活化障碍外,尽管果糖和乙醇在饱和乙醇中竞争性吸附,但发现Sn-SPP中催化部位的吸附也比Sn-Beta更为有利。沸石。































 京公网安备 11010802027423号
京公网安备 11010802027423号