当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NMR-Detected Dynamics of Sodium Co-Intercalation with Diglyme Solvent Molecules in Graphite Anodes Linked to Prolonged Cycling
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-09-10 , DOI: 10.1021/acs.jpcc.8b06089 Nicole Leifer 1 , Miryam Fayena Greenstein 1 , Albert Mor 1 , Doron Aurbach 1 , Gil Goobes 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-09-10 , DOI: 10.1021/acs.jpcc.8b06089 Nicole Leifer 1 , Miryam Fayena Greenstein 1 , Albert Mor 1 , Doron Aurbach 1 , Gil Goobes 1
Affiliation
|
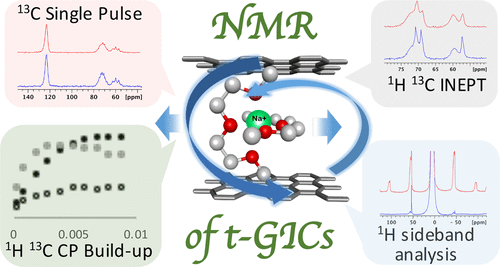
Following the success of Li-ion batteries, Na-ion batteries are becoming an important economical alternative, particularly where weight and density considerations are not of primary importance. Graphite, the anode of choice for nearly all commercial Li-ion battery applications, has only recently been successfully used as such in Na systems. This unprecedented success was due to the proper choice of solvent, e.g., diglyme. Interestingly, lithium performs poorly under such conditions, which is the converse of their respective behavior in standard carbonate solvents. These phenomena have been attributed to co-intercalation of the alkali ions upon their complexation with the smaller solvent molecules. In the case of Li, the use of such solvents leads to deterioration, while in the case of Na, it improves its electrochemical performance so substantially as to make the previously irrelevant Na–graphite system viable. Several studies have since followed, mainly focusing on the Na–diglyme intercalation; however, a thorough understanding of the mechanisms of ternary intercalation into graphite for both Na and Li is still lacking. In particular, the characteristic differences in location and dynamics of the guest complexes in the host material upon electrochemical cycling are not yet fully understood. In this study, the co-intercalation mechanisms of Na and Li in diglyme into graphite were explored via solid-state NMR spectroscopy. Direct evidence for the atomic proximity of both Na–(diglyme)2 and Li–(diglyme)2 complexes to the graphite planes in discharged electrodes was observed. Reduced mobility and stronger coupling of the Li–(diglyme)2 complex to the graphene electrons are seen, whereas higher mobility and weaker coupling to the host are detected for the Na–(diglyme)2 complexes in the galleries, providing molecular cues for the difference in cycling performance of the two systems.
中文翻译:

NMR检测到的石墨阳极中与二甘醇二甲醚溶剂分子共嵌入钠的动力学与延长的循环有关
随着锂离子电池的成功,Na离子电池正在成为一种重要的经济替代方案,尤其是在重量和密度方面的考虑不是最重要的情况下。石墨几乎是所有商用锂离子电池应用的首选阳极,直到最近才在Na系统中成功使用。这种空前的成功是由于溶剂的正确选择,例如,二甘醇二甲醚。有趣的是,锂在这种条件下的性能较差,这与它们在标准碳酸盐溶剂中的行为相反。这些现象归因于碱金属离子与较小溶剂分子的络合。在使用Li的情况下,使用此类溶剂会导致性能下降,而在使用Na的情况下,它极大地提高了其电化学性能,从而使以前不相关的Na-石墨系统可行。此后进行了几项研究,主要集中在Na-二甘醇二甲醚的插入方面。然而,对于Na和Li的三元嵌入石墨的机理仍然缺乏透彻的了解。尤其是,还没有完全理解在电化学循环时主体材料中客体配合物的位置和动力学的特征差异。在这项研究中,通过固态NMR光谱研究了二甘醇二甲醚中Na和Li的共嵌入机制。Na-(二甘醇二甲醚)的原子接近度的直接证据 然而,对于Na和Li的三元嵌入石墨的机理仍然缺乏透彻的了解。尤其是,还没有完全理解在电化学循环时主体材料中客体配合物的位置和动力学的特征差异。在这项研究中,通过固态NMR光谱研究了二甘醇二甲醚中Na和Li的共嵌入机制。Na-(二甘醇二甲醚)的原子接近度的直接证据 然而,对于Na和Li的三元嵌入石墨的机理仍然缺乏透彻的了解。尤其是,还没有完全理解在电化学循环时主体材料中客体配合物的位置和动力学的特征差异。在这项研究中,通过固态NMR光谱研究了二甘醇二甲醚中Na和Li的共嵌入机制。Na-(二甘醇二甲醚)的原子接近度的直接证据 通过固态NMR光谱研究了二甘醇二甲醚中钠和锂的共插入机理。Na-(二甘醇二甲醚)的原子接近度的直接证据 通过固态NMR光谱研究了二甘醇二甲醚中钠和锂的共插入机理。Na-(二甘醇二甲醚)的原子接近度的直接证据在放电电极中观察到2和Li–(二甘醇二甲醚)2与石墨平面的络合物。可以看到Li–(diglyme)2配合物与石墨烯电子的迁移率降低且偶联增强,而在画廊中的Na–(diglyme)2络合物检测到较高的迁移率且与主体偶联较弱,从而提供了分子提示。两种系统循环性能的差异。
更新日期:2018-09-11
中文翻译:

NMR检测到的石墨阳极中与二甘醇二甲醚溶剂分子共嵌入钠的动力学与延长的循环有关
随着锂离子电池的成功,Na离子电池正在成为一种重要的经济替代方案,尤其是在重量和密度方面的考虑不是最重要的情况下。石墨几乎是所有商用锂离子电池应用的首选阳极,直到最近才在Na系统中成功使用。这种空前的成功是由于溶剂的正确选择,例如,二甘醇二甲醚。有趣的是,锂在这种条件下的性能较差,这与它们在标准碳酸盐溶剂中的行为相反。这些现象归因于碱金属离子与较小溶剂分子的络合。在使用Li的情况下,使用此类溶剂会导致性能下降,而在使用Na的情况下,它极大地提高了其电化学性能,从而使以前不相关的Na-石墨系统可行。此后进行了几项研究,主要集中在Na-二甘醇二甲醚的插入方面。然而,对于Na和Li的三元嵌入石墨的机理仍然缺乏透彻的了解。尤其是,还没有完全理解在电化学循环时主体材料中客体配合物的位置和动力学的特征差异。在这项研究中,通过固态NMR光谱研究了二甘醇二甲醚中Na和Li的共嵌入机制。Na-(二甘醇二甲醚)的原子接近度的直接证据 然而,对于Na和Li的三元嵌入石墨的机理仍然缺乏透彻的了解。尤其是,还没有完全理解在电化学循环时主体材料中客体配合物的位置和动力学的特征差异。在这项研究中,通过固态NMR光谱研究了二甘醇二甲醚中Na和Li的共嵌入机制。Na-(二甘醇二甲醚)的原子接近度的直接证据 然而,对于Na和Li的三元嵌入石墨的机理仍然缺乏透彻的了解。尤其是,还没有完全理解在电化学循环时主体材料中客体配合物的位置和动力学的特征差异。在这项研究中,通过固态NMR光谱研究了二甘醇二甲醚中Na和Li的共嵌入机制。Na-(二甘醇二甲醚)的原子接近度的直接证据 通过固态NMR光谱研究了二甘醇二甲醚中钠和锂的共插入机理。Na-(二甘醇二甲醚)的原子接近度的直接证据 通过固态NMR光谱研究了二甘醇二甲醚中钠和锂的共插入机理。Na-(二甘醇二甲醚)的原子接近度的直接证据在放电电极中观察到2和Li–(二甘醇二甲醚)2与石墨平面的络合物。可以看到Li–(diglyme)2配合物与石墨烯电子的迁移率降低且偶联增强,而在画廊中的Na–(diglyme)2络合物检测到较高的迁移率且与主体偶联较弱,从而提供了分子提示。两种系统循环性能的差异。

































 京公网安备 11010802027423号
京公网安备 11010802027423号