Chemosphere ( IF 8.1 ) Pub Date : 2018-08-20 , DOI: 10.1016/j.chemosphere.2018.08.097 Zhiwen Cheng , Bowen Yang , Qincheng Chen , Yujia Tan , Xiaoping Gao , Tao Yuan , Zhemin Shen
|
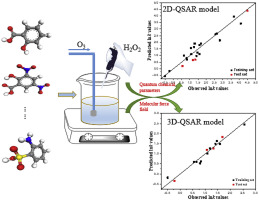
Synergistic oxidation of ozone (O3) and hydrogen peroxide (H2O2) is an effective water treatment for the elimination of organic pollutants. In this study, 23 organic compounds were conducted to study the reaction rate constants during O3-H2O2 oxidation. Then, two- and three-dimensional quantitative structure-activity relationship (QSAR) models were established to investigate the factors influencing the reaction rate constants by using multiple linear regression method and comparative molecular similarity index analysis (CoMSIA) method, respectively. Both of the two models showed good performance on predicting the reaction rate constants, the associated statistical indices of 2D-QSAR and 3D-QSAR models were = 0.898 and 0.952, = 0.841 and 0.951, = 0.968 and 0.970, respectively. But varied in the influence factors, as for the 2D-QSAR model, three quantum chemical parameters, included dipole moment, the largest change of charge in each atom during the nucleophilic attack, the maximum positive partial charge on a hydrogen atom linked with a carbon atom affected the reaction rate. While in the 3D-QSAR model, the electrostatic field played the most important role in evaluating the reaction rate with the contribution of 35.8%, followed by hydrogen bond acceptor and hydrophobic fields with the contribution of 24.9% and 23.2%, respectively. These two models provided predictive tools to study the influencing factors for the degradation of organics and might potentially be applied for estimating the removal properties of unknown organics in O3-H2O2 oxidation process.
中文翻译:

臭氧-过氧化氢氧化中有机化合物的反应速率常数的2D-QSAR和3D-QSAR模拟
臭氧(O 3)和过氧化氢(H 2 O 2)的协同氧化是消除有机污染物的有效水处理方法。在这项研究中,进行了23种有机化合物以研究O 3 -H 2 O 2期间的反应速率常数氧化。然后,建立了二维和三维定量构效关系(QSAR)模型,分别使用多元线性回归方法和比较分子相似性指数分析(CoMSIA)方法研究影响反应速率常数的因素。两种模型在预测反应速率常数方面均表现出良好的性能,2D-QSAR和3D-QSAR模型的相关统计指标分别为 = 0.898和0.952, = 0.841和0.951, 分别为0.968和0.970。但是影响因素各不相同,对于2D-QSAR模型,三个量子化学参数包括偶极矩,亲核攻击期间每个原子的最大电荷变化,与碳相连的氢原子上的最大正部分电荷原子影响反应速率。在3D-QSAR模型中,静电场在评估反应速率中起着最重要的作用,占35.8%,其次是氢键受体和疏水场,分别占24.9%和23.2%。这两个模型为研究有机物降解的影响因素提供了预测工具,并可能潜在地用于估算O 3 -H 2中未知有机物的去除性能。O 2氧化过程。

































 京公网安备 11010802027423号
京公网安备 11010802027423号