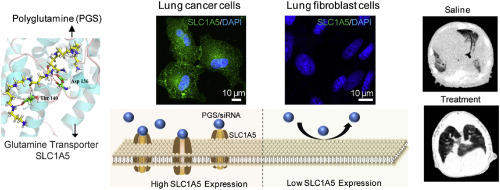
许多人类癌细胞对谷氨酰胺(Gln)具有致癌基因驱动的成瘾作用,因为与正常细胞相比,迅速增殖的癌细胞以显着增加的速率消耗Gln。因此,肿瘤细胞与宿主细胞竞争Gln,这导致Gln从正常组织流向肿瘤。在我们以前的工作中,我们已经开发出了Gln高分子类似物聚谷氨酰胺(PGS)并对其进行了表征,以用于传递基因调节剂,例如siRNA。在这里,我们假设PGS可以利用Gln转运蛋白SLC1A5特异性地将治疗性化合物递送至Gln上瘾的癌细胞。与人肺成纤维细胞HLF细胞相比,顺铂耐药的人肺腺癌A549 / DDP细胞显着过表达SLC1A5,SLC1A5与PGS具有高结合亲和力,这已通过分子对接分析证实。由于恶性细胞与正常细胞之间Gln代谢的差异,癌细胞中的PGS / siRNA复合物显着增加,特别是当细胞中缺乏Gln时,这反映了肿瘤微环境中常见的情况。此外,我们发现对Gln转运蛋白SLC1A5的化学和遗传抑制作用降低了PGS / siRNA复合物的细胞内在化,表明SLC1A5在细胞摄取PGS中起关键作用。反过来,PGS上调SLC1A5表达。PGS复合物摄取的增加会大大降低细胞内Gln水平。Gln降低导致细胞生长适度降低。为了恢复药物敏感性并进一步增强抗肿瘤作用,通过PGS递送系统施用了混合siRNA抗Survivin和抗MDR1(siSM)作为模型治疗剂,从而导致Survivin和MDR1的敲低,并进一步使癌细胞对药物顺铂(DDP)敏感。由于已施用PGS复合物iv主要在肺实质中积累,建立了肺原位肿瘤模型以评估其对肺肿瘤的抑制作用。PGS / siSM相当程度地降低了肿瘤的生长速度,而同时施用PGS / siSM和DDP则增强了这种效果,并没有明显改善其寿命。与我们的假设一致,该研究表明PGS模仿了SLC1A5途径中的Gln,并选择性地将治疗剂转移到了Gln上瘾的癌细胞中。我们的发现确定了一种基于Gln代谢的新型肺癌靶向策略,可以用作药物/基因递送系统。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Glutamine addiction activates polyglutamine-based nanocarriers delivering therapeutic siRNAs to orthotopic lung tumor mediated by glutamine transporter SLC1A5
Many human cancer cells exhibit an oncogenetic-driven addiction to glutamine (Gln) as rapidly proliferating cancer cells consume Gln at a dramatically increased rate compared to normal cells. Tumor cells, therefore, compete with host cells for Gln, which causes Gln to flux from normal tissues to the tumor. We have developed and characterized a Gln macromolecular analog polyglutamine (PGS) for the delivery of gene regulators, such as siRNAs, in our previous works. Here, we hypothesize that PGS can utilize the Gln transporter SLC1A5 to specifically deliver therapeutic compounds to Gln-addicted cancer cells. Compared to human lung fibroblast HLF cells, cisplatin-resistant human lung adenocarcinoma A549/DDP cells significantly overexpress SLC1A5, which has a high binding affinity to PGS, as confirmed through molecular docking analysis. Due to the differences in Gln metabolism between malignant and normal cells, PGS/siRNA complexes were remarkably increased in cancer cells, especially when cells were deprived of Gln, which mirrors the conditions that are commonly found in a tumor microenvironment. Furthermore, we identified that chemical and genetic inhibition of Gln transporter SLC1A5 reduced the cellular internalization of PGS/siRNA complexes, suggesting a critical role for SLC1A5 in PGS uptake in cells. In turn, PGS upregulated SLC1A5 expression. Increased uptake of PGS complexes profoundly decreased intracellular Gln levels. Decreased Gln caused a moderate reduction in cell growth. To restore drug sensitivity and further enhance anti-tumor effects, the hybrid siRNAs anti-Survivin and anti-MDR1 (siSM), as model therapeutics, were administered through the PGS delivery system, which resulted in knockdown of Survivin and MDR1 and further sensitized cancer cells to the drug cisplatin (DDP). Since PGS complexes administered i.v. mostly accumulated in the lung parenchyma, a lung orthotopic tumor model was established to evaluate their inhibitory effects on tumors in the lungs. PGS/siSM comparably decreased the rate of tumor growth, while concurrent administration of PGS/siSM and DDP enhanced this effect and insignificantly improved life span. Consistent with our hypothesis, this study demonstrated that PGS mimicked Gln in the SLC1A5 pathway and selectively ferried therapeutics to Gln-addicted cancer cells. Our findings identified a new lung cancer targeting strategy based on Gln metabolism and can be used as a drug/gene delivery system.

















































 京公网安备 11010802027423号
京公网安备 11010802027423号