European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-08-18 , DOI: 10.1016/j.ejmech.2018.08.043 Hui Zhao , Xiaoxia Hu , Kai Cao , Yue Zhang , Kuantao Zhao , Chunlei Tang , Bainian Feng
|
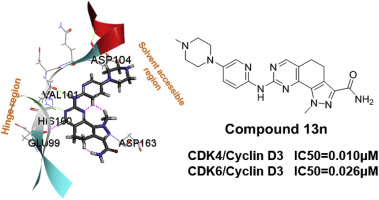
CDK4/6 pathway is an attractive target for development of anti-cancer drugs. Herein, we reported the design and synthesis of a series of 4,5-dihydro-1H-pyrazolo [4,3-h]quinazoline derivatives as selective CDK4/6 inhibitors. Applied with the optimizing strategy to the initial scaffold, it is found that compound 13n is able to selectively inhibit CDK4 and CDK6 with IC50 values 0.01 and 0.026 μM, respectively. The compound showed good anti-proliferative activity when tested in a panel of tumor cell lines with CDK4/6 related mechanism of action, the results clearly suggest that compound 13n works much better than Ly2385219 which is a selective CDK4/6 inhibitor. This compound was also found to have favorable pharmacokinetic parameters. Taken together, compound 13n could be selected for further preclinical evaluation.
中文翻译:

作为有效和选择性CDK4 / 6抑制剂的4,5-二氢-1H-吡唑并[4,3-h]喹唑啉衍生物的合成和SAR
CDK4 / 6途径是开发抗癌药物的有吸引力的靶标。在这里,我们报道了设计和合成一系列的4,5-二氢-1H-吡唑并[4,3-h]喹唑啉衍生物作为选择性CDK4 / 6抑制剂。将优化策略应用于初始支架,发现化合物13n能够选择性抑制CDK4和CDK6,IC 50值分别为0.01和0.026μM。当在一组具有CDK4 / 6相关作用机制的肿瘤细胞系中进行测试时,该化合物显示出良好的抗增殖活性,结果清楚地表明,化合物13n比选择性CDK4 / 6抑制剂Ly2385219更好。还发现该化合物具有有利的药代动力学参数。综合起来,复合可以选择13n进行进一步的临床前评估。






























 京公网安备 11010802027423号
京公网安备 11010802027423号