当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
GFAP Mutations in Astrocytes Impair Oligodendrocyte Progenitor Proliferation and Myelination in an hiPSC Model of Alexander Disease.
Cell Stem Cell ( IF 19.8 ) Pub Date : 2018-Aug-02 , DOI: 10.1016/j.stem.2018.07.009
Li Li , E Tian , Xianwei Chen , Jianfei Chao , Jeremy Klein , Qiuhao Qu , Guihua Sun , Guoqiang Sun , Yanzhou Huang , Charles D. Warden , Peng Ye , Lizhao Feng , Xinqiang Li , Qi Cui , Abdullah Sultan , Panagiotis Douvaras , Valentina Fossati , Neville E. Sanjana , Arthur D. Riggs , Yanhong Shi
Cell Stem Cell ( IF 19.8 ) Pub Date : 2018-Aug-02 , DOI: 10.1016/j.stem.2018.07.009
Li Li , E Tian , Xianwei Chen , Jianfei Chao , Jeremy Klein , Qiuhao Qu , Guihua Sun , Guoqiang Sun , Yanzhou Huang , Charles D. Warden , Peng Ye , Lizhao Feng , Xinqiang Li , Qi Cui , Abdullah Sultan , Panagiotis Douvaras , Valentina Fossati , Neville E. Sanjana , Arthur D. Riggs , Yanhong Shi
|
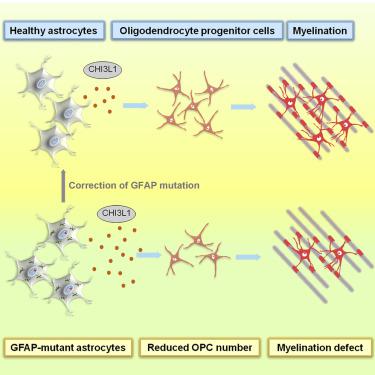
Alexander disease (AxD) is a leukodystrophy that primarily affects astrocytes and is caused by mutations in the astrocytic filament gene GFAP. While astrocytes are thought to have important roles in controlling myelination, AxD animal models do not recapitulate critical myelination phenotypes and it is therefore not clear how AxD astrocytes contribute to leukodystrophy. Here, we show that AxD patient iPSC-derived astrocytes recapitulate key features of AxD pathology such as GFAP aggregation. Moreover, AxD astrocytes inhibit proliferation of human iPSC-derived oligodendrocyte progenitor cells (OPCs) in co-culture and reduce their myelination potential. CRISPR/Cas9-based correction of GFAP mutations reversed these phenotypes. Transcriptomic analyses of AxD astrocytes and postmortem brains identified CHI3L1 as a key mediator of AxD astrocyte-induced inhibition of OPC activity. Thus, this iPSC-based model of AxD not only recapitulates patient phenotypes not observed in animal models, but also reveals mechanisms underlying disease pathology and provides a platform for assessing therapeutic interventions.
中文翻译:

星形胶质细胞中的GFAP突变会损害亚历山大疾病的hiPSC模型中少突胶质细胞祖细胞的增殖和髓鞘形成。
亚历山大病(AxD)是一种主要影响星形胶质细胞的白细胞营养不良,是由星形细胞丝基因GFAP的突变引起的。虽然星形胶质细胞在控制髓鞘形成中起着重要作用,但AxD动物模型并未概括关键的髓鞘形成表型,因此尚不清楚AxD星形胶质细胞如何促进白细胞营养不良。在这里,我们显示AxD患者iPSC衍生的星形胶质细胞概括了AxD病理学的关键特征,例如GFAP聚集。此外,AxD星形胶质细胞在共培养物中抑制人iPSC来源的少突胶质细胞祖细胞(OPC)的增殖,并降低其髓鞘形成潜力。基于CRISPR / Cas9的GFAP突变校正可逆转这些表型。AxD星形胶质细胞和死后大脑的转录组学分析确定CHI3L1是AxD星形胶质细胞诱导的OPC活性抑制的关键介质。因此,这种基于iPSC的AxD模型不仅概括了动物模型中未观察到的患者表型,而且揭示了疾病病理学的机制,并提供了评估治疗干预措施的平台。
更新日期:2018-08-17
中文翻译:

星形胶质细胞中的GFAP突变会损害亚历山大疾病的hiPSC模型中少突胶质细胞祖细胞的增殖和髓鞘形成。
亚历山大病(AxD)是一种主要影响星形胶质细胞的白细胞营养不良,是由星形细胞丝基因GFAP的突变引起的。虽然星形胶质细胞在控制髓鞘形成中起着重要作用,但AxD动物模型并未概括关键的髓鞘形成表型,因此尚不清楚AxD星形胶质细胞如何促进白细胞营养不良。在这里,我们显示AxD患者iPSC衍生的星形胶质细胞概括了AxD病理学的关键特征,例如GFAP聚集。此外,AxD星形胶质细胞在共培养物中抑制人iPSC来源的少突胶质细胞祖细胞(OPC)的增殖,并降低其髓鞘形成潜力。基于CRISPR / Cas9的GFAP突变校正可逆转这些表型。AxD星形胶质细胞和死后大脑的转录组学分析确定CHI3L1是AxD星形胶质细胞诱导的OPC活性抑制的关键介质。因此,这种基于iPSC的AxD模型不仅概括了动物模型中未观察到的患者表型,而且揭示了疾病病理学的机制,并提供了评估治疗干预措施的平台。































 京公网安备 11010802027423号
京公网安备 11010802027423号